
Slides for this Exercise: 24, 1205, 7, 11, 35, H9190, VM 45, VM46, FH132, VM43, MCO0039, MCW023
THERE ARE PRE-RECORDED LECTURES FOR THIS EXERCISE
Please go to the listing page for these to download and view them
Introduction to the Lymphatic Tissues
Spleen
Lymph Nodes
Thymus
The lymphatic system is vital to the defense against illness. If infectious agents manage to breach the mechanical barriers and gain entry to the milieu interieur, the cells which deal with the invasion are those which have arisen, developed, matured, and/or been stored in lymphatic organs. Changes in the histology of lymphatic organs are important diagnostic criteria.
For reasons that should be obvious, the lymphatic system is intimately related, both structurally and functionally, to the blood vascular system. Lymph itself is a clear, slightly yellowish and opalescent fluid derived from blood. It contains white blood cells, specifically lymphocytes. Certain specialized organs devoted to processing and modifying lymph and lymphoid cells are present in all normal mammals. Lymph starts as blood fluid that is "strained" into the tissue spaces under the hydrostatic pressure of the pumping blood. It's drained from the intercellular spaces by thin vein-like lymphatic vessels, re-entering the venous circulation carrying lymphocytes that enter the stream at certain specific points.

Just as a refresher, here's a lymphocyte (L), as it appears in circulating blood (H&E) about 400x; and in a smear stained with Wright's stain ("Diff-Quik") at 1000x. Lymphocytes in circulation are quiescent, and their cytoplasm is very reduced. Their nuclear : cytoplasmic ratio is very high, and almost the entire volume is filled with the nucleus. The nuclear chromatin is compacted and coiled up (since the cell isn't making protein or dividing) and therefore stains very densely. In the tissues, once they leave the circulation, lymphocytes take on different appearance. One tissue-resident manifestation is the plasma cell. This is an "activated" B lymphocyte, i.e., one that has met its designated antigen and is engaged in making antibodies to it. Activation and transformation of lymphocytes occurs only in tissue spaces, not in circulation.
It isn't possible to tell by looking at a smear or an ordinary tissue section whether this is a B or T lymphocyte; morphologically they're indistinguishable. You need special immunological labeling for specific surface markers to tell them apart. However, in the lymphatic organs we'll examine in this exercise, based on information from non-morphological studies, it's now well established that certain locations have B cells, certain ones have T cells, and some have both. We'll discuss these in the context of the different organs, specifically the spleen; lymph and hemal nodes; the thymus; tonsils; the aggregated lymphatic nodules of the digestive tract (commonly called Peyer's patches) and their avian equivalent, the bursa of Fabricius.
The nomenclature for lymphatic tissue is confusing, not least because there are several systems in use. In an attempt to clarify it I'll use two basic terms: diffuse and nodular lymphatic tissue. Diffuse lymphatic tissue consists of any unorganized collection of lymphocytes. These can be found wherever localized conditions have attracted lymphocytes in large numbers, and vary greatly in size. Such aggregations are usually transient features. The diffuse lymphatic tissue found in lymphatic organs is present all the time, however.
Nodular lymphatic tissue is always found surrounded by diffuse tissue, and it's much more organized. The typical example of nodular lymphatic tissue is the germinal center, a highly ordered collection of B-lymphocytes found in some (not all) lymphatic organs (see below). Not all lymphatic organs will contain germinal centers. Germinal centers never occur outside of those lymphatic system organs that can provide an appropriate environment for them. Thus, lymphatic tissue outside of specifically lymphatic organs is, by definition, diffuse in nature. Nodular tissue always occurs within a background of diffuse tissue. Diffuse lymphatic tissue can be found outside of lymphatic system organs. For example, the aggregation of lymphocytes in a gland in response to invasion by bacteria is legitimately considered diffuse lymphatic tissue. In these cases nodular tissue wouldn't be found in it.
(By the way, don't confuse the terms "nodular lymphatic tissue" and "lymph node" with each other. The lymph node is a specific anatomical organ you can dissect out of an animal. The node contains diffuse lymphatic tissue and in most cases, nodular lymphatic tissue—in the form of germinal centers—as well.)

 Here you see both diffuse and nodular tissue. This is from slide 672; it's a lymph node. Compare it to the diagram. On this slide you'll be able to make a number of distinct, organized aggregations of lymphocytes with a "light pole" and a "dark pole" or "cap" where the cells are more densely packed. These are germinal centers; a lymph node is one place where these occur. Germinal centers are nodular lymphatic tissue. They're polarized and organized, and though you can't see it in this type of section, they're also demarcated from the general "background" of the rest of the lymphocytes by specialized cells.
Here you see both diffuse and nodular tissue. This is from slide 672; it's a lymph node. Compare it to the diagram. On this slide you'll be able to make a number of distinct, organized aggregations of lymphocytes with a "light pole" and a "dark pole" or "cap" where the cells are more densely packed. These are germinal centers; a lymph node is one place where these occur. Germinal centers are nodular lymphatic tissue. They're polarized and organized, and though you can't see it in this type of section, they're also demarcated from the general "background" of the rest of the lymphocytes by specialized cells.
The "background" is of course diffuse lymphatic tissue; a motley agglomeration of lymphocytes loafing around, passing through the node on their way to somewhere else, and taking a break from the labor of saving the body from infection. (We'll discuss how they get in and out below.) This association of nodular/diffuse, germinal center/unorganized tissue can be found not only in lymph nodes, but in most (not all) other organs of the lymphatic system. To sum up: lymphatic regions organized into recognizable structures inside a lymphatic organ are "nodular" and everything else is "diffuse" lymphatic tissue.
The spleen is really part of the circulatory system, but it's always described with the lymphatic organs because of the very large population of lymphocytes found in it. The spleen is a flaccid bag that serves as a storage site for blood, a processing station for the scavenging of aged erythrocytes, and a few other things. It's one of the "dispensable" organs, because mammals get along quite nicely without a spleen. In the case of a traumatic injury that ruptures the spleen, the easiest thing to do is to take it out, and a splenectomy is commonly done. It's easier to remove the spleen than to try to repair it and risk renewed hemorrhage.
These are two of those troublesome terms that really applicable in gross anatomy, but with which the histologist has to deal as well. Oh, well...here goes:
Cut a freshly-removed spleen across and it looks like a field of dark red material with white spots in it. On the basis of its gross appearance in fresh sections, the spleen is traditionally said to have the bulk of its parenchyma as red pulp, with isolated areas of white pulp interspersed through it.
The red pulp gets its appearance from the formed elements of the blood (mostly erythrocytes) it contains. The white pulp consists almost entirely of lymphocytes, in a peculiar association with the arterial blood supply (see below). These two terms are quite logical when applied to gross specimens, but things become a little confusing when they're applied to microscopic sections. Briefly, in terms of microscopic sections, "white pulp" is equivalent to the lymphocyte population of the spleen, in the form of the periarteriolar lymphocyte sheath or PALS (see below). "Red pulp" is everything else, which means the splenic cords and the sinuses between them.
OK, I got that over with...
 This low magnification image from slide 1023 shows both the "red" and "white" pulp. "Red pulp" fills the bulk of the spleen's volume. White pulp is the blue stained areas visible within it. You're really seeing aggregated masses of cells. The pink stained strands running through the field are the connective tissue septae or trabeculae (T) that subdivide the spleen's interior volume. They arise from and are continuous with the capsule (C). The capsule is mainly collagenous fibrous CT, but there's a good bit of smooth muscle in it, too. This allows the spleen to contract and eject stored erythrocytes when needed. The magnification here is low enough (about 20x) that you can make out the red and white pulp pretty much as you would in a fresh gross preparation.
This low magnification image from slide 1023 shows both the "red" and "white" pulp. "Red pulp" fills the bulk of the spleen's volume. White pulp is the blue stained areas visible within it. You're really seeing aggregated masses of cells. The pink stained strands running through the field are the connective tissue septae or trabeculae (T) that subdivide the spleen's interior volume. They arise from and are continuous with the capsule (C). The capsule is mainly collagenous fibrous CT, but there's a good bit of smooth muscle in it, too. This allows the spleen to contract and eject stored erythrocytes when needed. The magnification here is low enough (about 20x) that you can make out the red and white pulp pretty much as you would in a fresh gross preparation.
The "PALS" labeled here is the periarteriolar lymphocyte sheath, the characteristic association of lymphocytes and blood vessels in the spleen (see below). The lymphocytes are arranged along the arteries forming a sleeve or sheath. Collectively, the PALS is the "white pulp." The rest of the interior volume of the spleen is the splenic cords, collectively constituting the "red pulp," and the blood sinuses between them.
Splenic Circulation
Any description of the spleen's histology must necessarily be centered around the arrangements of its blood vessels. After you've studied that, move on to see the actual structures on slide 1023.
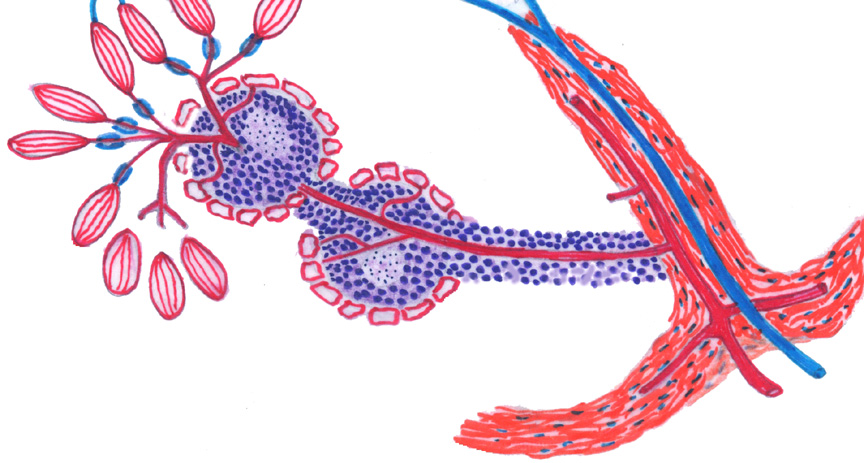 This sketch shows the layout of the blood supply to the spleen, and is well worth getting firm in your mind before you begin examining actual specimens, it will make it much easier to comprehend what you see on the slides.
This sketch shows the layout of the blood supply to the spleen, and is well worth getting firm in your mind before you begin examining actual specimens, it will make it much easier to comprehend what you see on the slides.
Blood enters from the splenic arterial supply, via the splenic capsule, at lower right; and the breakup of arteries into capsular and trabecular segments begins almost immediately. As is true in other organs, the angiogenic properties of the CT capsule are are needed to create the routes for the blood supply entering and leaving the spleen. The main input, the splenic artery, ramifies in the capsule and sends branches deeper and deeper. Blood leaving the organ is drained back through a series of veins in the septa and the capsule, eventually exiting via the splenic vein. Both the artery and vein are grossly located at the hilus.
As soon as a branch of the arterial input reaches the interior space of the spleen, it acquires the periarteriolar lymphocyte sheath, or PALS, which it retains almost to the smallest subdivisions. The PALS is a place in which the special conditions required for proliferation of B lymphocytes can be met, and at intervals there will be germinal centers along it. Germinal centers are individually transient and depend on the immune state of the animal, as they do in other parts of the immune system; but their presence is a feature of the spleen in all species to some extent. The arterial vessels that have been covered with lymphocytes are the central arteries.
Eventually the arteriolar supply subdivides to the point where the PALS becomes attenuated to a few cells, or even lost altogether. These small arteries tend to run in bundles and hence are termed penicillar arteries, from their resemblance to the hairs of a paintbrush (the Latin word for a paintbrush is penicillum). The role of the penicillar arteries is to deliver the incoming blood to the red pulp (which is not shown in this sketch) i.e., the splenic cords and sinuses. They're sometimes called "arteries of the red pulp." Some segments of the penicillar arteries acquire a peculiar "sheath" of phagocytic cells in their walls, and have been termed sheathed arterioles in the short regions where this occurs. The presence of a sheath is inconstant and there are species variations; its role is unclear, as well.
Beyond this point what happens to the blood is a matter of debate. Some authorities contend that the blood enters more or less directly into the sinuses of the red pulp. Others contend that there is no direct connection: that the arteriolar side simply ends and that erythrocytes enter the sinuses from the cords. These are, respectively the "closed" and "open" theories of splenic circulation. Whatever happens, though, the blood is eventually collected from the sinuses into actual veins and then routed back through the trabecular veins to the splenic vein and thence to the general circulation.
Splenic Capsule
Returning now to slide 1023, you can see that there's a distinct capsule around the spleen. It's mostly collagenous and elastic connective tissue, interspersed with a fair amount of smooth muscle. One function of the spleen is to act as a reservoir of erythrocytes, and the contraction of the capsule expels these into circulation when needed. The admixture of muscle is particularly pronounced in ruminants and horses. Animals that depend on running for their survival tend to have rather muscular splenic capsules: they can store erythrocytes there and release them into the general circulation when needed for extra oxygen carrying capacity. "Blood doping" a racer by a transfusion just before the race gives him more erythrocytes; a practice that, properly done, is nearly undetectable. Animals evolved to live at low altitudes often have oxygen loading curves that depend on a high partial pressure of atmospheric oxygen. When moved to high elevations, they often generate extra erythrocytes to compensate for the thin air. These extra erythrocytes are stored in the spleen, to be released when needed to deal with exertion.
The capsule sends septae or trabeculae part way into the interior volume of the organ, and from these arise a delicate network of reticular fibers, to form a stromal network on which the lymphocytes sit. These are very difficult to see in H&E stained specimens, but with appropriate special stains you can see them.
PALS: The Periarteriolar Lymphocyte Sheath
As an arteriole leaves a septum and enters the interior volume of the spleen it immediately acquires a continuous coating of lymphocytes. This "sleeve" of lymphocytes is the periarteriolar lymphocyte sheath, or PALS. You can see it on slide 1023 quite well as basophilic areas with small blood vessels running through them. These central arteries are the first part of the arterial input into the spleen. All of the PALS collectively constitute what the gross anatomist calls the spleen's "white pulp."
The PALS is a continuous feature of the arterial supply, almost to the point where it breaks up into capillaries. The main blood vessel you see running through most profiles of the PALS is the central artery, and it continues to branch. Eventually, the branching reaches the point where the PALS is reduced to only one or two cells on the smallest portions of the arteries formed by subdividing the central arteries.
Here are two views from slide 1023, one at about 100x and another about 400x, to show the relationship of the PALS to the arterial supply. You can see the PALS in any orientation, from cross section (as if you were looking into a tube of lymphocytes, as at right) to longitudinal. The central artery is in the middle in either case. This PALS and the red pulp around it are easily differentiated.
The PALS is diffuse lymphatic tissue, but within it germinal centers frequently develop. Careful examination of the right image will show the dark "cap" of one of these. Now, the PALS, being diffuse, has a loose stromal framework of reticular fibers made by a variant for of fibroblast. The resident lymphocytes of the diffuse region of the PALS are supported by it, like birds sitting on telephone wires: this sort of arrangement is typical of all diffuse lymphatic tissue. Due to the dense packing of the lymphocytes in the PALS you will not be able to make out the fibers in an H&E preparation, even in the diffuse parts. But special staining for reticular fibers will reveal it pretty easily.
Within the germinal centers, however, things are different. The germinal center is a special place: it's a clone of B-lymphocytes, and it has to be isolated so that these cells can develop properly. Consequently the stroma in germinal centers isn't fibrillar in nature. Instead, special cells of a different cell line than the fibroblasts form a stroma within the germinal center and support the B cells in it. A similar non-fibrillar stroma is characteristic of the thymus, and will be discussed below.
Continuation of the Splenic Blood Supply Beyond the Central Arteries
 The central arteries, like any self-respecting artery, break up into smaller
ones eventually. Each one gives rise to a tuft of small vessels, the penicillar
arteries. The
PALS is almost wholly gone by the time this level of branching is reached.
You're looking at them cut in cross section. These are very small vessels, and the PALS around them is almost nonexistent.
The central arteries, like any self-respecting artery, break up into smaller
ones eventually. Each one gives rise to a tuft of small vessels, the penicillar
arteries. The
PALS is almost wholly gone by the time this level of branching is reached.
You're looking at them cut in cross section. These are very small vessels, and the PALS around them is almost nonexistent.
Sheathed Capillaries
Upon reaching the red pulp and finally losing the last of the PALS, some of the penicillar arteries individually break up into a peculiar form of capillary. These are the sheathed capillaries, the name referring to the presence of a specialized region of the capillary wall.
While most blood vessels are lined with simple squamous epithelium, the sheathed capillaries are an exception. The endothelium of the sheathed capillaries is composed of fusiform cells, oriented parallel to the long axis. These specialized capillaries are surrounded by a sheath of reticulocytes and macrophages, bound together by reticular fibers. Not all of the capillaries arising from the penicillar arteries have such a sheath. Those that do have special properties, and the cells of the wall are phagocytic. You can often find them by looking for lipofuscin in the walls of the sheathed portions.
Past the sheath, the capillaries return to the normal configuration expected of these vessels. Sheathed capillaries may be found on slides 673 and 1023. Not all species have this type of blood vessel.
We have now followed the arterial supply as far as we can, and it's time to look at the venous side of the system.
The red pulp constitutes the bulk of the splenic volume. The supporting reticulum of the red pulp is continuous with the septa. Again, it consists of reticular fibers invested by reticulocytes.
If you look carefully at the red pulp, it will become clear that it's arranged
into anastomosing splenic cords, separated by venous sinuses. The
sinuses are blood spaces. They're lined with endothelium and contain
circulating blood cells. Hence most of what you find in them is erythrocytes.
The splenic cords are the masses of cells in between the sinuses. They contain a lot of erythrocytes, but they also contain many other cell types: macrophages most prominently. It's in the cords that the phagocytosis of the aged erythrocytes occurs, and consequently a stain for iron (such as that used on slide 674) will reveal the presence of hemosiderin in the cords very well.
This specimen shows the cord and sinus arrangement of the red pulp quite well. The sinuses contain a few erythrocytes and the normal circulating white cells of the blood; it is essentially a blood vessel. It can be difficult to see the sinuses clearly: often the spleen contracts on death (or anesthesia) and the sinuses get gorged with blood. Since there are so many erythrocytes in the cords, it can be hard to discern the boundary.
However, like all blood vessels, the sinuses are lined by cells: In this case the cells in the lining are phagocytic. On slide 673 (left above) and 674 (right) you can make out bits of brownish material in them, defining the limits of the sinus. One of the functions of the spleen is the degradation of senescent erythrocytes: at the end of their useful lives (90 to 120 days) erythrocytes develop cell surface associated "senescence antigens" to mark them as the legitimate prey of the macrophages in the cords of the red pulp. They're seized, phagocytosed, and the salvageable components of their compounds recycled, like old cars being dismantled in a junkyard. The leftover part, hemosiderin can build up in the spleen considerably over long periods of time.
In slide 674, stained with Prussian Blue, the hemosiderin that's produced in the process of dismantling hemoglobin is seen as blue blotches. note that these are in the cords. The cords are the location of numerous splenic macrophages with this specific task. The phagocytic lining cells of the sinuses are less selective, and in them you'll see lipofuscin, the breakdown product of cellular scavenging.
In most spleen sections, this cord-and-sinus separation is very difficult to see, but if you can find an area of slide 1023 in which the density of cells is not too great, you should be able to decipher the cord-sinus-cord arrangement without too much trouble. One clue as to which is which on this slide is the ratio of erythrocytes to other cell types. In the sinuses, the erythrocytes are present in the same overwhelming proportion that they represent in the blood. In the cords, however, there are many more non-erythrocytes present, and the nuclear density is much higher than in the sinuses. Look also for the endothelium which separates cords from the adjacent sinuses.
Since the sinuses are blood vessels, they're lined by endothelium, the cells of which are fusiform in shape and oriented longitudinally with respect to the long axis of the sinus. The wall of the sinus, while it demarcates the cells in the cords from the blood flowing through the sinuses, is nevertheless compliant enough to permit passage of cells through it. Cells of the blood can and do move from a sinus to an adjacent cord, and vice versa, and the static impression given by microscopic sections is incorrect. The endothelium lining the splenic sinusoids is phagocytic. The sinusoids eventually join together to form veins of the red pulp, which in turn coalesce into larger veins in the septa. Drainage of the entire organ is through the large vein running parallel to the splenic artery at the hilus.
Well, there is no avoiding it any more: Having accounted for the arterial flow and the venous drainage, it's necessary to deal with the question of the connection between the two sides.
The termination of splenic capillaries is a matter of debate. It's believed by some investigators that the capillaries of the spleen end by opening right into the splenic cords, and that blood thus released passes through the cords and into the adjacent sinuses. This would mean that there is no direct connection between the arterial and venous sides, and that the cords in essence act as "filters" through which the total volume of blood has to pass before returning to the venous circulation. This is the open theory of splenic circulation.
An alternative view is that the capillaries do in fact end by joining the sinuses directly, as is the case in other organs in which sinusoids are found. Self respecting arteries, after all, should end in capillary beds. Thus the circulatory loop is closed, and the cells in the splenic cords get there from the blood passing through the sinusoids. This is, of course, called the closed theory.
The arguments for and against both theories rest on theoretical and empirical grounds. The Open Theory proponents point out that if one cannulates the splenic artery, and pumps in dyes, the dyes will be found in both the cords and the sinusoids. But if one cannulates the vein, the dyes are only found in the sinusoids. Therefore, they argue, there is no direct connection, as otherwise the cords and the arteries leading into them would be full, too.
The adherents of the Closed Theory reply that there would be more or less constant hemorrhage if the Open Theory were correct, as the ending of arteries in parenchymal tissue could not result in anything else, in the absence of venous drainage capable of handling the large volumes of blood the spleen holds. To the evidence of the dyes, they respond by saying that splenic veins are so delicate that it is impossible to retro-perfuse them without damage, and that a failure to get dyes into the arteries simply means the connections between the two vessel types was broken by the pressure of perfusion.
As you probably expected, there is a third theory arguing that both types of ending exist simultaneously. That at times the circulation is closed, and others open; that in normal times the open system operates, but when there is need for those reserve erythrocytes, the connections between arteries and veins takes over, the system is closed, and the blood is pumped out.
You pays your money and takes your choice.
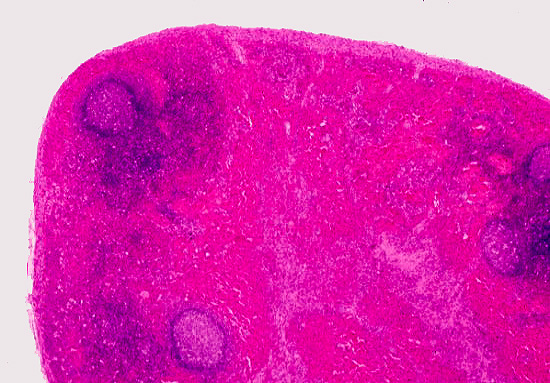 Hemal nodes are characterized by the presence of large numbers of erythrocytes. Lymph nodes (see below) and hemal nodes share some similarities of structure, but really are
fundamentally different organs both qualitatively and quantitatively.
Hemal nodes are characterized by the presence of large numbers of erythrocytes. Lymph nodes (see below) and hemal nodes share some similarities of structure, but really are
fundamentally different organs both qualitatively and quantitatively. The lymph node is the most organized of the lymphatic organs. Grossly, they're shaped like beans, with a depression on one side, the hilus. Blood vessels enter and leave the lymph node at the hilus, but lymphatic vessels enter at the periphery, and exit at the hilus. More will be said about the flow of lymph through the node below.
Lymph nodes are found along larger lymphatic vessels. The lymphatic vessels, remember, drain tissue fluid back towards the venous circulation. Lymphatic vessels originate as small "blind" structures, i.e., there is no closed loop as there is for the blood circulation. Flow of lymph through these vessels isn't under a continuous pressure the way the blood is; it's "squeezed" back by contraction of muscles around the vessels. The lymph nodes are stationed along these routes to act as "filters" for lymph as it passes through.
Lymph is pushed through from the periphery of the node to its center, and then continues on its way back to join the venous circulation. Since lymph nodes are pretty sizable organs (most of them are about the size of a pea or larger, some as big as a large broad bean) they need blood vessels and an internal circulation of their own. Blood vessels enter and leave them without releasing blood into the volume of the node, any more than blood is released into other organs.
Here's a lymph node at low power, from slide 672. The node is a discrete structure: histologically it's 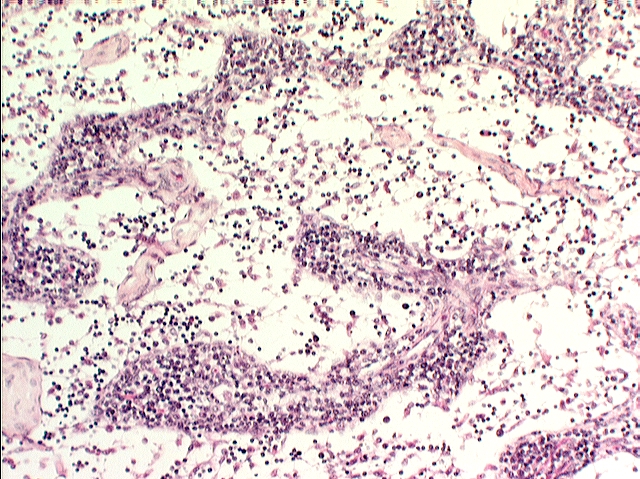 possible to distinguish a cortex and a medulla. In the cortex you find the bulk of the lymphatic tissue, both diffuse and organized into germinal centers, several of which are visible in this specimen. The medulla, by contrast, has much more loosely spaced lymphocytes, with open spaces between them. This forms a cord-and-sinus type arrangement, allowing lymph to flow through the sinuses, between the cords of cells. You can see this more clearly in the specimen at left, taken at high power; it shows only the medullary region. The medulla contains lymphocytes but not germinal centers. Macrophages are present scattered throughout the parenchyma, in both regions: they can usually be identified by their content of lipofuscin.
possible to distinguish a cortex and a medulla. In the cortex you find the bulk of the lymphatic tissue, both diffuse and organized into germinal centers, several of which are visible in this specimen. The medulla, by contrast, has much more loosely spaced lymphocytes, with open spaces between them. This forms a cord-and-sinus type arrangement, allowing lymph to flow through the sinuses, between the cords of cells. You can see this more clearly in the specimen at left, taken at high power; it shows only the medullary region. The medulla contains lymphocytes but not germinal centers. Macrophages are present scattered throughout the parenchyma, in both regions: they can usually be identified by their content of lipofuscin.
To delineate details of the stroma,
use slide 29, which is stained with Wilder's reticulum method. It will clearly
show you the fine fibrils that make up the supporting scaffold of this organ. The easiest way to visualize the interior architecture of the node, and its stroma, is to image a box filled with strings stretching throughout its interior. these "strings" are the collagen fibers. They arise from the capsule and the trabeculae, and the lymphocytes are sitting on them. They're made by a fibroblast-like cell, in the same way as any other collagen fiber.
Capsule and Stroma
Lymph nodes have a discrete CT capsule which sends trabeculae deep into the volume of the organ. The capsule acts as an overall envelope for the node, and also as the route of entry for lymphatic vessels. It's composed of dense irregular collagen with a few elastic fibers as well. In ruminants there is a considerable admixture of smooth muscle.

Compare the diagram and the micrograph above with slide 672. The image on the right is from a specimen stained with the Mallory's routine, and shows the capsule and a small trabecula very clearly. A delicate CT stroma arises off the trabeculae; fibers of the stroma rise from and are anchored to the trabeculae and to the inner face of the capsule. The fibers form a complex webwork that crisscrosses the greatest part of the volume of the node, especially in the cortical region. These fibers also traverse the subcapsular space and the sinuses. Notice also that there's considerable space immediately under the capsule, and surrounding the trabeculae: this space is important to the flow of lymph through the node.
Lymph Flow
 At the periphery and in the region around the outside of the node you
should be able to locate at least one afferent lymphatic vessel. These bring lymph to the periphery of the node, penetrate into and through the capsule, and release the lymph into the subcapsular space. Many if not most of these will have valves in them.
Valves are a feature of most of the larger lymphatic vessels, and are closer
together than in veins. Note that the valve cusps in the peripheral vessels point towards the node: flow is one-way. Flow of lymph through the node is from the periphery towards the hilus.
At the periphery and in the region around the outside of the node you
should be able to locate at least one afferent lymphatic vessel. These bring lymph to the periphery of the node, penetrate into and through the capsule, and release the lymph into the subcapsular space. Many if not most of these will have valves in them.
Valves are a feature of most of the larger lymphatic vessels, and are closer
together than in veins. Note that the valve cusps in the peripheral vessels point towards the node: flow is one-way. Flow of lymph through the node is from the periphery towards the hilus.
Lymph, like blood in the venous side of the circulatory system, is moved along by contractions of muscles and movements of visceral organs that act to squish the floppy walls of the lymph vessels and "squeeze" the lymph towards the nodes. Obviously, some system has to be in place to arrange for the lymph to be squeezed the right way, lest it simply slosh around and never get back into the circulation. So the valves are present. Movement to and through nodes in progressively larger lymph vessels takes place, until eventually the lymph reenters the blood.
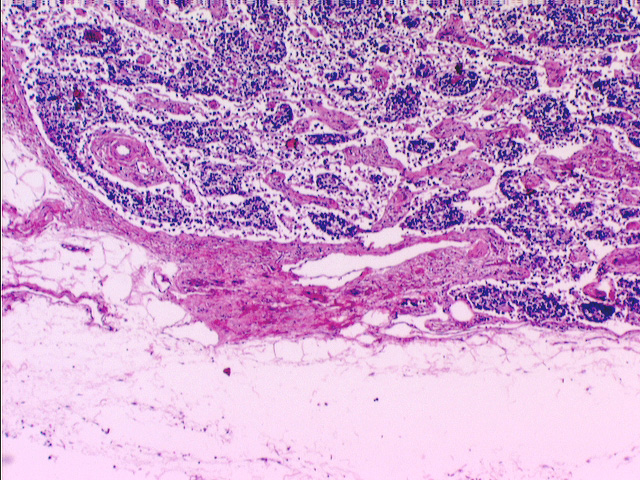 Immediately beneath the capsule is a large subcapsular space
in communication with the rest of the volume of the node, allowing the incoming lymph to trickle through the cortex into the more open sinuses
of the medulla. Passage of lymph through the larger vessels and towards a node is accomplished
by contractions of the adjacent musculature and movements of the viscera.
Compression of the very thin walls forces lymph through the valve, and then to
and eventually through the node. Typically there are a few lymphocytes present in an afferent vessel.
Immediately beneath the capsule is a large subcapsular space
in communication with the rest of the volume of the node, allowing the incoming lymph to trickle through the cortex into the more open sinuses
of the medulla. Passage of lymph through the larger vessels and towards a node is accomplished
by contractions of the adjacent musculature and movements of the viscera.
Compression of the very thin walls forces lymph through the valve, and then to
and eventually through the node. Typically there are a few lymphocytes present in an afferent vessel.
The drainage of the node is via a coalescence of the sinuses, which form an efferent lymphatic vessel at the hilus. Again, there are often valves present, but since the vessel is leaving the node, they point away from it. This vessel leaves the node in company with the blood vessels.
The valve in the afferent vessel will be visible on slide 672 as a small flap, (actually they are paired flaps) connected to the walls of the vessel. Like the valves of veins, the valves of lymphatic vessels are designed to permit only one-way flow. This situation is analogous to rest areas on an interstate highway: traffic must enter and leave going in one direction.
Blood Flow in Lymph Nodes
The flow of blood through the node enters via an arteriole at the hilus, is
distributed throughout the volume of the node, and leaves via a vein, also at
the hilus. Entering blood vessels run through the CT septa peripherally, towards the outer
region of the node, where they break up. They supply blood to the medullary
cords and the cortex, both of which have capillary plexuses. Areas of nodular
lymphatic tissue will be enveloped individually by dedicated capillary
plexuses.
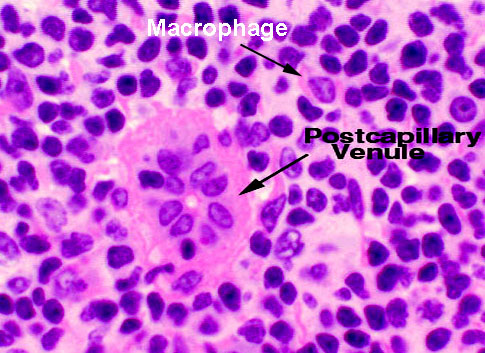 In the return circuit, there are special regions of postcapillary venules in the cortex. The postcapillary venules are devoid of a muscular tunic, and
have specialized endothelial lining cells. These are the site at which small
lymphocytes carried in the blood enter the node. The lining epithelium of the
postcapillary venules is cuboidal rather than squamous, and apparently has
recognition antigens on it so that the lymphocytes can find it. Lymphocytes are
capable of insinuating themselves between the endothelial cells in this region,
and entering the parenchyma. Look for what appears to be a small blood vessel
with a high cuboidal lining. Note that the direction of migration is the reverse of that in the thymus (see below):
lymphocytes brought to the node migrate out of the blood and into
the node's volume. They will return to the circulation via the lymphatic
vessels. In the case of the thymus, lymphocytes that are maturing in the organ
migrate out to the blood.
In the return circuit, there are special regions of postcapillary venules in the cortex. The postcapillary venules are devoid of a muscular tunic, and
have specialized endothelial lining cells. These are the site at which small
lymphocytes carried in the blood enter the node. The lining epithelium of the
postcapillary venules is cuboidal rather than squamous, and apparently has
recognition antigens on it so that the lymphocytes can find it. Lymphocytes are
capable of insinuating themselves between the endothelial cells in this region,
and entering the parenchyma. Look for what appears to be a small blood vessel
with a high cuboidal lining. Note that the direction of migration is the reverse of that in the thymus (see below):
lymphocytes brought to the node migrate out of the blood and into
the node's volume. They will return to the circulation via the lymphatic
vessels. In the case of the thymus, lymphocytes that are maturing in the organ
migrate out to the blood.
After the region of the postcapillary venules, the venules enter the medullary cord and regain a normal lining; small venules coalesce to form the outflow from the node at the hilus.
The thymus is the primary lymphatic organ of mammals, and its presence and functionality is required for immunocompetence to be fully established. In one strain of mice, nude, the thymus is congenitally absent, and these animals must be housed in a sterile environment, as they have no functional immune system.
Structure of the Thymus

Begin with slide 117, a thymus from a young pig. A low-power view is shown above. It's covered by a thin connective tissue capsule, and lobulated by septa originating from this. The thymic lobules are clearly divided into a dark staining cortex and a lighter medulla by the differential density of the cells in each region. The cell types in these areas are the same, but their distribution within the lobules isn't uniform, and there are important physiological differences between the two populations.
This shows the distinct lobulation of the organ. You won't find germinal centers in the thymus: it's a sort of "training academy" for the T-lymphocytes, so what you have here are T-lymphocytes that have not yet become immune competent (in the cortex) or have just "graduated" and have started out as rookie T-cells. While the thymus is an important immune organ, it's not a site of antibody production. Hence there can be no germinal centers.
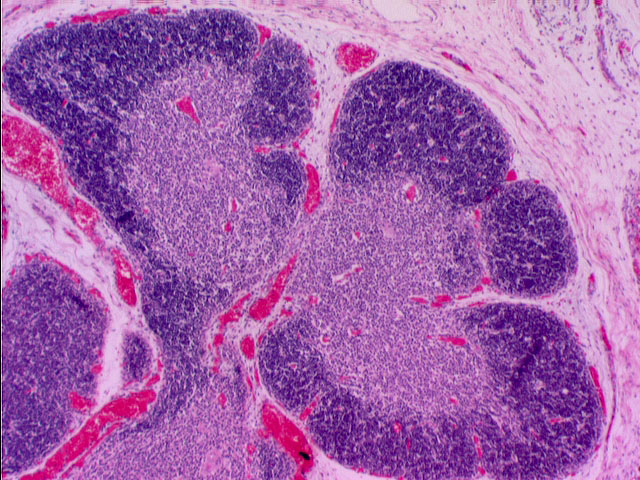 This somewhat higher magnification shows two lobules. The darkly stained cortex and lighter medulla are obvious. The staining difference is attributable to the much higher density of T-cells in the cortex.
This somewhat higher magnification shows two lobules. The darkly stained cortex and lighter medulla are obvious. The staining difference is attributable to the much higher density of T-cells in the cortex.
The thymic lobules are well demarcated by CT, but they are actually all interconnected. If you were to enter the cortex at the point you see here, you could move from any place in it to any other place in the cortex of this organ freely. The lobulation, in other words, is incomplete. So the septation of the thymus isn't complete, as it is in some other lobulated organs. Serial sectioning would easily show that the different lobes and lobules are continuous with each other through bridges of parenchymal material. Blood vessels enter the thymus through the capsule, and travel along septa to the corticomedullary border, at which point they enter the parenchyma. Blood vessels are pretty obvious in this image. On close examination you can see them not only between lobules, but as small capillary beds and venules in the parenchyma. Arterioles entering the thymus send capillaries to the cortex, which branch at the periphery and return. At the corticomedullary junction, postcapillary venules are found, which represent specialized sites of transit of matured lymphocytes into the blood.
 The thymus is relatively largest (in relation to body weight) very
early in life, and actually continues to grow in absolute size for a while after birth.
Nevertheless, in the normal course of aging, the thymus undergoes involution,
by which the total volume of active tissue is reduced. It's replaced by
connective tissue and adipose tissue for the most part, but some functional
tissue remains throughout life. Many stimuli (such as overexposure to radiation
and some chemicals) can cause accidental involution, and in these cases
the organ is usually capable of regeneration if the offending stimulus is
removed.
The thymus is relatively largest (in relation to body weight) very
early in life, and actually continues to grow in absolute size for a while after birth.
Nevertheless, in the normal course of aging, the thymus undergoes involution,
by which the total volume of active tissue is reduced. It's replaced by
connective tissue and adipose tissue for the most part, but some functional
tissue remains throughout life. Many stimuli (such as overexposure to radiation
and some chemicals) can cause accidental involution, and in these cases
the organ is usually capable of regeneration if the offending stimulus is
removed.
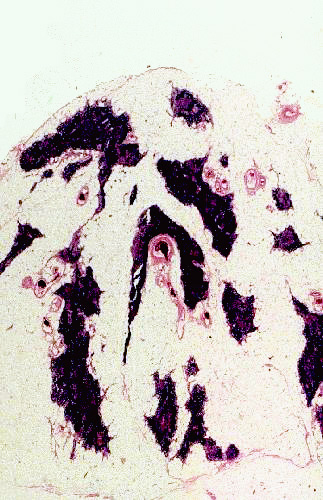
This example of an involuted thymus is from an elderly cat. Involution is a normal process. As an animal ages, the functional parenchyma of the organ is replaced with fat and connective tissue, and the organ as a whole diminishes in size and as a proportion of body weight. It does not become totally nonfunctional, however. As more and more T-lymphocytes become "trained" there is less need for the thymus, but even late in life it may be necessary to produce immunocompetent T-cells; so the parenchyma never completely disappears and it always retains some level of function. Natural involution, as in this specimen, is an irreversible process. But accidental involution due to some exogenous agent, such as chemical or radiation insult, can usually be reversed once the offending stimulus is removed.
The thymus has three principal cell types: thymocytes, i.e., lymphocytes of the thymus; a few macrophages; and the specialized epithelio-reticular cells, stellate cells which provide a supporting framework (the stroma) for the parenchyma.
Most of the lymphocytes in the thymus are T-cells. In order to acquire immunocompetence, these cells must reside in the thymus for a period of time. Macrophages are interspersed among these, and can be seen as larger cells with vacuolar nuclei. Frequently coarse granules of undigested material may be present in the macrophages' cytoplasm.
The epithelio-reticular cells are almost impossible to demonstrate in H&E preparations, being obscured by the lymphocytes that sit on them. Hence you will not see them in your specimens as such, even though they are indeed present.
The thymic stroma isn't fibrillar in nature. It is wholly cellular. The epithelio-reticular cells are branching and tree-like in shape, and put forth processes, supported by intracellular cytoskeletal elements. These processes come into contact with each other, and are held together at the points of contact with desmosomes. The processes thus form a completely cellular scaffolding—sometimes called a cytoreticulum to distinguish it from other, truly fibrillar, reticula—to support the rest of the parenchyma.
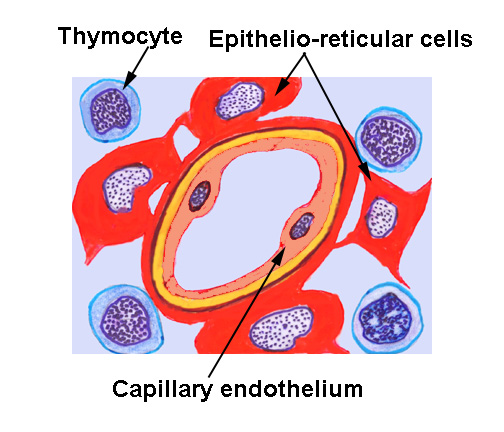 Most importantly to normal thymic function, these cells form a physical, cellular
boundary between the parenchymal thymocytes and the rest of the body. At the periphery of the cortex, subjacent to the capsule, and around all blood
vessels, the processes form a continuous cellular layer constituting the blood-thymus
barrier. In concert with the lining epithelium of the capillaries, the barrier prevents exposure of the immature lymphocytes to blood borne antigens.
Most importantly to normal thymic function, these cells form a physical, cellular
boundary between the parenchymal thymocytes and the rest of the body. At the periphery of the cortex, subjacent to the capsule, and around all blood
vessels, the processes form a continuous cellular layer constituting the blood-thymus
barrier. In concert with the lining epithelium of the capillaries, the barrier prevents exposure of the immature lymphocytes to blood borne antigens.
The thymus has to be maintained as an immunologically protected site; the cells here are undergoing maturation and specialization, and to subject them to the influences of the outside world may mean they "learn" to respond incorrectly. You will recall that a similar "cytoreticulum" arrangement is present in the germinal centers where B-lymphocytes proliferate. Like germinal centers, the thymus is a special place and it's isolated from the possibly malign influences of the outside in the same way.
 Even though you probably won't be able to see functional ones in your slide, epithelio-reticular cells eventually undergo degeneration, and you'll easily find their remnants. The exhausted cells organize themselves into
concentric eosinophilic whorls of material, called thymic corpuscles (or Hassall's
corpuscles, after Arthur H. Hassall, 1817-1894, an English physician).
These are of unknown function, and appear very early in life. They appear to be
nothing more than debris, nonfunctional degenerative remains. Each consists of a concentric mass of epithelial cells joined by desmosomes and containing intracellular bundles of intermediate filaments. They may reach 100 µm in diameter. Thymic corpuscles only are found in mammals. Their accumulation seems to be related to age.
Even though you probably won't be able to see functional ones in your slide, epithelio-reticular cells eventually undergo degeneration, and you'll easily find their remnants. The exhausted cells organize themselves into
concentric eosinophilic whorls of material, called thymic corpuscles (or Hassall's
corpuscles, after Arthur H. Hassall, 1817-1894, an English physician).
These are of unknown function, and appear very early in life. They appear to be
nothing more than debris, nonfunctional degenerative remains. Each consists of a concentric mass of epithelial cells joined by desmosomes and containing intracellular bundles of intermediate filaments. They may reach 100 µm in diameter. Thymic corpuscles only are found in mammals. Their accumulation seems to be related to age.
A few of what might be called "miscellaneous lymphoid organs" are scattered in various places in the body. While unorganized masses of diffuse lymphatic tissue can be found in almost any CT or any epithelial organ, in these "miscellaneous" organs it has a regular organization into nodular lymphoid tissue, often showing germinal centers.
One such place is the tonsils. Slide 116 is a section of palatine tonsil, and the presence of these organized structures is obvious. Tonsils are found in association with the oral cavity, and can easily be identified by their surface covering of stratified squamous epithelium. The gross structure of a tonsil is what you might get if you pushed your extended fingers into modeling clay: deep crypts, with lymphoid tissue filling the spaces between them.
This is a typical tonsil; the association of lymphatic tissue and an overlying stratified squamous epithelium are the identifying histological features. Tonsils are usually anatomically well defined by the presence of a CT investment on the attachment side; germinal centers are normally present. The mucus type glands below the lymphatic tissue are small salivary glands whose ducts open onto the surface of the tonsil. Tonsils are usually well encapsulated by CT on the side away from the oral cavity, making them easy to remove if they become infected. The cell types are the same as those seen in other lymphoid organs, including lymphocytes and macrophages and plasma cells.
Slide 849 is a longitudinal section of the ileum segment of the small intestine. In the submucosal parts of this organ (the CT layer separating the lining epithelium from the outer muscular coat) there are large aggregates of lymphoid tissue. These are the aggregated lymphatic nodules, classically called Peyer's patches (after Johann K. Peyer, 1653-1712, a Swiss anatomist).
These structures are large enough to be visible with the naked eye as whitish areas on that side of the intestine opposite to its mesenteric attachment. In ruminants, they have germinal centers present in them almost from the time of birth. They're the site of maturation and development of the B-cells in mammals )as the thymus is for T-cells).
This cross section of the ileum nicely displays the aggregated lymphatic nodules (still universally called "Peyer's Patches" despite the official nomenclature rules against eponyms). These are the sites of maturation and development of B-lymphocytes. They're very prominent structures in most species. It's quite common to find germinal centers in them, though as the animal ages the number of germinal centers decreases. In cattle, for example, germinal centers are present at the time of birth and they decline relatively rapidly with age, as the pool of "memory" lymphocytes increases.
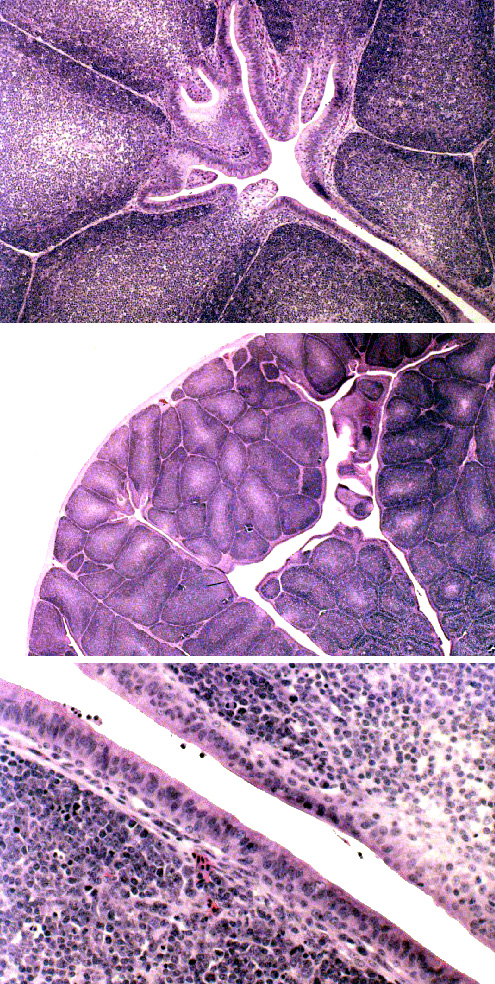 The bursa of Fabricius, seen on slide 675, is an organ peculiar to birds.
(It's named for Giralamo Fabrizi, 1533-1619, an Italian anatomist, who, as was
customary among men of science in those days, Latinized his name). This is a
blind, sac-like structure on the caudodorsal side of the cloaca. The term
"bursa" is the Latin for "purse" and implies the blind
"pocket book" gross structure of this organ.
The bursa of Fabricius, seen on slide 675, is an organ peculiar to birds.
(It's named for Giralamo Fabrizi, 1533-1619, an Italian anatomist, who, as was
customary among men of science in those days, Latinized his name). This is a
blind, sac-like structure on the caudodorsal side of the cloaca. The term
"bursa" is the Latin for "purse" and implies the blind
"pocket book" gross structure of this organ.
Like the tonsil, it is a number of blind, crypt-like invaginations, and some references actually call it a "cloacal tonsil," which isn't so far off the mark. Its appearance is at first glance similar to the thymus, and in some ways it performs a thymus-like function; it's involved in conferring immunocompetence on the avian equivalent of B-cells in birds. The aggregated lymphatic nodules of the ileum are the mammalian equivalent of this organ.
The free surface of the avian bursa is covered with stratified columnar epithelium, not stratified squamous, which makes it easy to distinguish from tonsils or the thymus. Most of the cells in it are lymphocytes.
This image shows three views of the bursa, to show its layout and general features. At low magnification (center image) it could also easily be mistaken for a mammalian thymus. But the lack of extensive primary lobulation and the scanty CT investment would rule out this "look alike" if you were trying to identify it on a microscope slide. Furthermore, the thymus has no overlying epithelium. It might be mistaken for a tonsil: but the overlying epithelium (bottom image) is columnar, not squamous.
The bursa is considered to be the equivalent of the aggregated ileal nodules in mammals: a place where B-lymphocytes must reside for a while to become completely immune competent.