
 In this exercise we'll be concerned mainly with mature bone, its structural
variations, and the way in which it's produced. Macroscopically, anatomic bones
are categorized as compact or spongy, based on their gross
appearance.
In this exercise we'll be concerned mainly with mature bone, its structural
variations, and the way in which it's produced. Macroscopically, anatomic bones
are categorized as compact or spongy, based on their gross
appearance. SLIDES USED IN THIS EXERCISE: 18, 18A, 17, VM05, 98, 80, VM01, VM10, 79, 81
THERE ARE PRE-RECORDED LECTURES FOR THIS EXERCISE
Please go to the listing page for these to download and view them
Bone
Principles of Osteogenesis
Joints and Tendons
Bone Marrow and Hemopoiesis
Bone, like other connective tissues, has cells, fibers, and a matrix. In bone, however, the extracellular matrix is calcified, and the fibers (which are collagen) are very highly ordered. The cellular component is vital to bone function, as we'll see, but in terms of the total volume, it's a comparatively small proportion.
Bone is a truly remarkable structural material, with properties that make it ideal for its functions of structural and physiological support. It's relatively light, but it has very high tensile and compressive strength, and a good deal of elasticity. There is a scene in the motion picture Never Cry Wolf in which the protagonist holds up a rib bone from an elk, then snaps it in two with his bare hands. Try this sometime; if you can't get hold of an elk rib, a beef rib will do. Superman would have trouble performing this trick, and the average person would have trouble doing it to a sheep rib, let alone one from an elk. It's obvious that the script writers were more concerned with drama than reality!
One other misconception about bone is that it's a static tissue. Nothing could be further from the truth; it's about the most dynamic material in the body. Bone is a reservoir for calcium and phosphorus, and there is a constant flux of minerals in and out of it. Bone responds to injury by rapid healing. It can be made to grow in different ways by clever tricks of orthodontia and surgery (the braces you may once have worn were physically affixed to your teeth, but their purpose was really to remodel the bone of the jaw), and it's greatly affected by nutritional and metabolic changes. Bones are continually being modified, reshaped, remodeled, and overhauled.
Bone has considerably more collagen content than does cartilage. The collagen fibers are much more orderly in their arrangement as well. Elastic fibers are minimal or absent. Perhaps as much as 25-30% of the total organic material in a bone is collagen. The collagen fibers give bones the ability to resist snapping and breaking. They act in some respects the way the cords in a tire sidewall do.
The mineral component, which gives bone its hardness, is chiefly a form of calcium phosphate, called hydroxyapatite. The trigger for the crystallization of this material is not known, but it may involve the interaction of collagen fibers with the GAGs of the matrix material. Matured bone contains about 65% mineralized matter, the rest being collagen and matrix. The mineral gives bone its toughness and rigidity, the highly ordered fibrous component provides tensile strength and flexibility.

 In this exercise we'll be concerned mainly with mature bone, its structural
variations, and the way in which it's produced. Macroscopically, anatomic bones
are categorized as compact or spongy, based on their gross
appearance.
In this exercise we'll be concerned mainly with mature bone, its structural
variations, and the way in which it's produced. Macroscopically, anatomic bones
are categorized as compact or spongy, based on their gross
appearance.
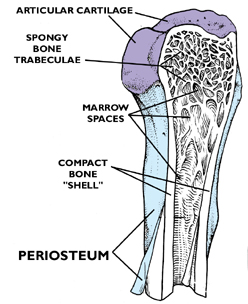
Consider first the macroscopic appearance of a typical long bone. The image at left is a typical example, a sketch of the knee joint. The articular ends of the long bones are covered with cartilage; each bone (including the patella) is composed of a hard "shell" of mature, fully developed bone: i.e., lamellar bone, so called because of the organization of its compact substance. Inside the hollow ends of each of the long bones is the trabecular network of spongy or cancellous bone. These flat, interlocking trabeculae are also lamellar, but arranged in somewhat of a different way than the denser compact bone of the shaft. The greater part of a long bone is the shaft, or diaphysis.The outer surface of the shaft is covered with a tough collagenous CT, the periosteum. Some of the cells in it have osteogenic capabilities, and are derived from the same stem line as the fibroblasts. The periosteum is analogous to the perichondrium, and it's tightly adherent to the surface of the bone.
If the bone is from a young animal that's still
growing, there should be a cartilaginous growth plate at one or both
ends. You can see such a bone at right: the growth plate is the place where replacement of cartilage by bone occurs in the process of endochondral ossification. Anatomically this is the epiphysis of the bone. Elongation of the anatomic bone occurs at the epiphyses (there's a second epiphysis at the other end). Cartilage proliferation in these areas occurs rapidly: fast enough to keep ahead of the ever-encroaching wave of ossification. Hence the anatomic bone continues to elongate. At puberty, hormonal changes occur. Secretion of growth hormone declines (this hormone targets the epiphyseal growth plates) resulting in reduced proliferation. Eventually, the ossification process catches up and overtakes it, causing the obliteration of the growth plate. This event, "closure" of the epiphyses, means no further increase in length can occur. In humans this happens around the age of 18. In domestic animals it varies considerably with species: epiphyseal closure in canines is pretty much complete by 18 months' age; in cattle it takes about 2-1/2 years. The process of closure is sequential: the distal growth plate is removed first, and then the proximal one. The details of endochondral ossification, and the mechanics of bone elongation, will be discussed below.
The outer surface of the shaft is covered with a tough collagenous CT, the periosteum. Some of the cells in it have osteogenic capabilities, and are derived from the same stem line as the fibroblasts. The periosteum is analogous to the perichondrium, and it's tightly adherent to the surface of the bone.
Microscopic Appearance of Anatomic Bones
Now let's consider the histology of mature bone, and consider how compact and spongy bone differ in their organization. Keep in mind that both compact and spongy bone are "lamellar" forms! The lamellae are arranged differently, and differ in overall density and robustness, but both are very organized in a structural sense. In this respect they differ from "woven" bone, the type initially laid down in osteogenesis.
We'll start with compact bone, and consider the arrangement of it in cross section and longitudinal section. You will find it represented in slide 18. Examine it first at low power. This is a piece of dried bone cut into a wafer with a diamond saw. It's from the shaft of a long bone, and it's histology is pretty typical. You will note there appear to be circular areas, and between these there are less regularly shaped regions. These are, respectively, the osteons and the interstitial systems of compact bone.

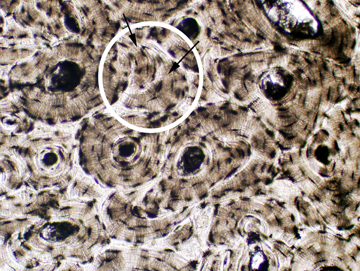
Here are two examples of compact bone, cut in cross section. As you can see, there are discrete areas. Some are organized as concentric rings around a small opening, easily seen in the left image. These circular profiles are the osteons; compact bone showing this organization is referred to as osteonal bone. The central osteonal canals are visible here as dark holes, more or less round. They're the route for the blood vessels and nerves serving the osteons. The branches that run between adjacent osteons are passed through lateral canals.
Bone remodeling is a continuous process, with osteons constantly being built, removed, and replaced. The irregularly shaped areas outside of the circular osteons (one of which is highlighted in the right-hand image) are interstitial systems. These are the remnants of old osteons, varying in size and shape depending on how old they are and how many new osteons have been organized around them. An interstitial system might or might not have an osteonal canal, depending on how long ago the process of removing and replacing it began. The proportion of osteons to interstitial systems is fairly predictable with increasing age, making it possible to age a piece of bone fairly accurately this way. This is a matter of some importance in forensic medicine.
The osteon is the basic structural unit of compact bone. In three dimensional view, an osteon is a
cylinder composed of 4 to 20 concentric lamellae arranged around a central
opening, the osteonal canal. A useful analogy to help understand the nature of the osteon is a roll of
paper towels: both are arranged as layers of thin material (the lamellae) around a relatively open central core. In cross section,
as you see them in slide 18, each osteon appears to be a series of concentric
rings, like the growth rings on a tree. This is actually deceptive, because each osteon extends the length of the anatomic bone; most of the compact bone of the outer shell is osteons and their associated interstitial systems. In the center
of this field is a single osteon, with parts of one or two more around it. The long, cylindrical osteon has 3-20 layers or lamellae, oriented around the
 central osteonal canal. Between the hardened lamellae the resident cells of bone, osteocytes, are entrapped in lacunae. The lacunae (singular is "lacus") are spaces produced when the matrix surrounding the cell was hardened. From each lacuna radiate thin canaliculi, tiny channels through the hard substance. The canaliculi are visible here as thin hair-like projections radiating from the lacunae.
central osteonal canal. Between the hardened lamellae the resident cells of bone, osteocytes, are entrapped in lacunae. The lacunae (singular is "lacus") are spaces produced when the matrix surrounding the cell was hardened. From each lacuna radiate thin canaliculi, tiny channels through the hard substance. The canaliculi are visible here as thin hair-like projections radiating from the lacunae.
Anatomic bones are very heavily vascularized, and the osteonal canals are major channels through which pass blood vessels, lymphatic vessels, and nerve fibers. Adjacent osteons are linked together by lateral canals which connect the osteonal canals. Thus the osteonal and lateral canals form a complicated and ramified set of channels through the dense material of the bone. At the outer and inner surfaces of the anatomic bone, the blood vessels running through them can emerge onto the surface or into the marrow cavity. Canaliculi are an important part of this system for distribution of nutrients and removal of wastes. It's impossible for materials to diffuse through the hardened bone matrix of the lamellae, and not all of the osteocytes are lucky enough to be immediately adjacent to a blood vessel. The canaliculi provide a route by which processes from one osteocyte can contact those of adjacent ones: via the canalicular system, all osteocytes are potentially in communication with one another. They pass information, nutrients, and/or wastes from cells near blood vessels to the ones trapped in lacunae far from "civilization."
These two images show nice examples of lateral canals and their relationship to the osteonal canals. At left the plane of section has passed through a large osteon (O). A lateral canal (LC). is running through the wall of the osteon, and is in communication with the osteonal canal (OC). In the lower image are several more such connections. In living tissue, the canals you see in these specimens—both osteonal and lateral—would be occupied by blood and lymphatic vessels, and nerves. In a preparation of this type, the cellular and soft-tissue elements are destroyed, and only the hard, dead material remains behind.

We've been looking at osteons in cross section, but if you make a section that's parallel to the long axis of the osteon, as in the diagram and photomicrograph at left, the relationship between the lateral canals and the osteonal canals is clarified. In this orientation, the osteonal canal is seen running vertically; and the lateral canals connect adjacent ones. Notice also the ubiquitous canaliculi running through the hard substance.
The diagrammatic representation of the blood supply in an anatomic bone (above) shows how the osteonal/lateral canal system begins at the surface, at a point where a blood vessel penetrates through a nutrient foramen. The blood vessels ramify via the canal system throughout the compact bone, eventually bringing blood into the marrow cavity via perforating canals. In the marrow spaces, the blood vessels communicate with the blood sinusoids. Not only is there normal supportive blood circulation, it's via the osteonal/lateral canals that newly formed elements of the blood exit the marrow and get into the general circulation.
The canaliculi are the final level in the distribution of nutrients and waste materials, and to an individual osteocyte trapped in a lacuna, they are vital to survival.
 The osteocytes function to keep the bone in good condition. Since bone is lamellar
the lacunae are located
between adjacent lamellae; hence they're neatly arranged into rows, which
coincide with the division between one lamella of the osteon and the next. Given that between osteocyte and the next there's hard, calcified
bone matrix, and quite a few collagen fibers,
there can't be any diffusion. But osteocytes have very long processes: processes of adjacent osteocytes
remain in contact by passing through the minute canaliculi. The canaliculi radiate outward from each lacuna, each one filled by an
osteocyte process which contacts
that of another osteocyte. At the points of contact gap junctions allow for a
continuous cell-to-cell pathway for nutrients and waste
products through the otherwise impermeable hard substance of the bone. It's a kind of "bucket brigade" that keeps the isolated osteocytes in tenuous contact with the outside world.
The osteocytes function to keep the bone in good condition. Since bone is lamellar
the lacunae are located
between adjacent lamellae; hence they're neatly arranged into rows, which
coincide with the division between one lamella of the osteon and the next. Given that between osteocyte and the next there's hard, calcified
bone matrix, and quite a few collagen fibers,
there can't be any diffusion. But osteocytes have very long processes: processes of adjacent osteocytes
remain in contact by passing through the minute canaliculi. The canaliculi radiate outward from each lacuna, each one filled by an
osteocyte process which contacts
that of another osteocyte. At the points of contact gap junctions allow for a
continuous cell-to-cell pathway for nutrients and waste
products through the otherwise impermeable hard substance of the bone. It's a kind of "bucket brigade" that keeps the isolated osteocytes in tenuous contact with the outside world.
The spider-like quality of the osteocyte lacunae and their radiating canaliculi is evident here. Each canaliculus carries a fine osteocyte process; at the points of contact of these processes, gap junctions between the adjacent plasma membranes are the sites of interchange of materials and information between the cells. This system is vital to the survival of compact osteonal bone. Canaliculi are not extensively developed in the flat plates of cancellous (spongy) bone because the plates are thin and diffusion can occur between the osteocyte and the nearby marrow space.
 Now transfer your attention back to slide 79. This slide is the hip joint of a fetal mouse. This is a synovial joint, and you should study its architecture carefully. A joint like this is a sealed unit filled with fluid: the hip joint is a "ball-and-socket" with the "ball" being the rounded end of the femur, neatly fitted into the "socket" of the fixed bone. The articulating surface of the ball, and the apposed surfaces of the socket, are all covered with hyaline cartilage, and bathed in the fluid of the joint. The fluid provides lubrication, exactly the way grease fills the ball joints of your car's steering gear.
Now transfer your attention back to slide 79. This slide is the hip joint of a fetal mouse. This is a synovial joint, and you should study its architecture carefully. A joint like this is a sealed unit filled with fluid: the hip joint is a "ball-and-socket" with the "ball" being the rounded end of the femur, neatly fitted into the "socket" of the fixed bone. The articulating surface of the ball, and the apposed surfaces of the socket, are all covered with hyaline cartilage, and bathed in the fluid of the joint. The fluid provides lubrication, exactly the way grease fills the ball joints of your car's steering gear.
The joint is sealed off from the outside world by dense irregular CT forming a flexible cover firmly anchored around the edges. (This arrangement is exactly like the dust seal that protects the ball joint of a car. (Note that here again we see the exception to the rule that cartilage is always covered by perichondrium; where the articular surface is exposed to synovial fluid, the cartilage covering it is "naked.")
Let's examine the cancellous bone located inside the round articular end and in the bones forming the "socket" into which this femoral head fits.
Cancellous bone is lamellar, but it doesn't form the orderly osteons and interstitial systems of compact bone. Instead it forms sheets and plates, which in sections are seen as anastomosing trabeculae (from Latin, trabus = beam) and spicules.
The spaces between the trabeculae are continuous with the main marrow cavity in the diaphysis and contain marrow. Both the marrow cavity and the spaces among the trabeculae are lined with a very thin layer of mixed cell types, the endosteum; some of these cells are osteogenic, and some osteolytic. We'll look at them in detail below. The trabeculae can be thought of as a mosaic of angular pieces, each covered with endosteum.
If you examine the trabeculae at medium magnification, you can verify that they have osteocytes enclosed within lacunae, and these lacunae are arranged in orderly rows. Osteons don't form here, however. Since the trabeculae are quite thin, the osteocytes are nourished by diffusion from the bone marrow via the canaliculi, and there is no need for the osteonal system, with its tortuous channels to carry nutrient vessels.
Nor is there much need for compressive strength, as in long bones. The osteons of compact bone are a structural adaptation to provide mechanical strength under vertical loads, and the spongy portions of the bone aren't subjected to this kind of stress at all.
Although we've discussed osteocytes, we haven't yet dealt with the other major cellular components of bone, the osteoblasts and the osteoclasts, both of which are seen on slides 16 and 17.
 The osteoblasts are the bone forming cells. As the "-blast" root word implies, these are active "builders" of bone. Osteoblasts, are capable of making any fibers and/or matrix materials appropriate to their locations. Under the right circumstances, osteoblasts can be differentiated out of existing precursors (see below).
The osteoblasts are the bone forming cells. As the "-blast" root word implies, these are active "builders" of bone. Osteoblasts, are capable of making any fibers and/or matrix materials appropriate to their locations. Under the right circumstances, osteoblasts can be differentiated out of existing precursors (see below).
In the trabecular portions of slide 16, you'll see plenty of osteoblasts. They're basophilic, more or less cuboidal in shaped, ranged in rows along the pieces of bone and the inner surface of the marrow spaces. They're close to the bone they're making, which is usually less densely stained than the cells.
Osteoblasts are strongly basophilic because they're making proteins: collagen fibers, laid down in precise orientation; and also the proteoglycan matrix material. Osteoblasts are polarized cells; that is, they have an architecture in which different structures are located at each end, based on the cell's activity. They're usually polarized so that the nucleus is away from the bone they're working on. In this image you'll also note that some osteocytes have become partially or completely enclosed in lacunae: when this happens they complete their differentiation to enter the "maintenance mode" i.e., they become osteocytes. If later they are released from their imprisonment it's believed that they can return to being osteoblasts again. Thus the osteoblast and the osteocyte are really the same cell in two separate physiologic states.
 Osteocytes not only differentiate out of existing precursor cells in the marrow cavity, they arise by differentiation of stem cells in the bone's CT covering, the periosteum. This happens whenever the outside surface of the bone has to be re-shaped or modified. For example, as the bone elongates, the wall is thinned from within and built up from the periosteal surface, to keep it from becoming too thick. And of course as the fetus develops the bone not only elongates, it changes shape and develops various tubercles and projections on its surface (every one of which has a name and every one of which you have to learn in Gross Anatomy...) This is accomplished by appositional growth, just as cartilaginous structures are modified.
Osteocytes not only differentiate out of existing precursor cells in the marrow cavity, they arise by differentiation of stem cells in the bone's CT covering, the periosteum. This happens whenever the outside surface of the bone has to be re-shaped or modified. For example, as the bone elongates, the wall is thinned from within and built up from the periosteal surface, to keep it from becoming too thick. And of course as the fetus develops the bone not only elongates, it changes shape and develops various tubercles and projections on its surface (every one of which has a name and every one of which you have to learn in Gross Anatomy...) This is accomplished by appositional growth, just as cartilaginous structures are modified.
Osteoblasts actively synthesize the bone matrix material and the collagen fibers. Later they'll harden the matrix by depositing apatite, entrapping and transforming themselves into the osteocytes we've already looked at. Although the process of lacuna formation and "entrapment" of the cells is similar to what happens in cartilage, unlike chondrocytes, osteocytes don't divide once they're locked inside their lacunae. Consequently, bone doesn't have isogenous groups.
Obviously, if the process of making new bone were not counteracted by the removal of some existing bone, the marrow cavity would diminish in size with time, and the numerous projections on the surface would not appear in the right places. Removal of existing bone is the job of the osteoclast, also seen on slides 16 and 17.
The osteoclast is much larger than the osteoblasts, and is usually multinucleated. Look for these cells on the ends of spicules of the trabeculae, or in pockets they've created for themselves on the surface of a spicule. In the EM, osteoclasts have a conspicuous ruffled border (and thus enhanced surface area). The osteoclasts secrete hydrogen ions into the extracellular space to solubilize the mineralized matrix, and also produce hydrolytic enzymes (including collagenase) to deal with the fibrous components. In addition to its role in sculpting anatomic bones, resorption releases calcium and phosphorus from the mineralized matrix into the circulation for other purposes. Disease, dietary deficiencies, metabolic disorders, hormonal problems, or prolonged calcium deprivation result in increased release of calcium and increased porosity of the bone, rendering it susceptible to mechanical damage.
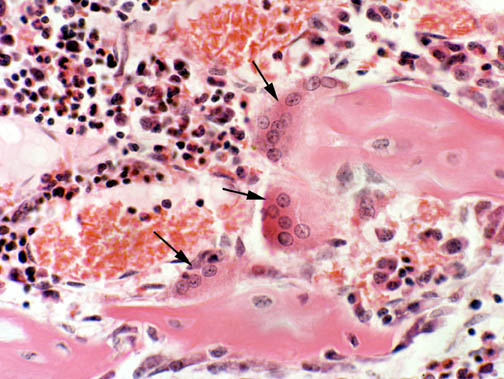
In this image three big osteoclasts are sitting on a piece of bone in the center of the field Osteoclasts are multinucleated: one shown here has at least nine nuclei, which is not a very unusual situation. Some have fewer and some have more, depending on the requirements of the time and place where they're formed.
The osteoclast does not originate from the osteoblast line. Recently the precursor cell of the osteoclast has been identified as a blood-borne element: radiolabeling has shown that osteoclasts, monocytes, and macrophages all have a common precursor cell. The precursor can "choose" which path it will take, and some "choose" to differentiate into osteoclasts. In the blood, these precursors structurally are seen as monocytes.
Enzymatic staining can be be used to distinguish a subpopulation of monocytes with the enzyme complement characteristic of the osteoclasts. In the bone, the mon-nucleated cells complete their differentiation and combine to form the multinucleated, active osteoclast. Because it's formed by the aggregation of pre-existing single cells the osteoclast is a syncytium, a multinucleated protoplasmic mass derived by fusion of mononuclear precursors.
Since osteoclasts arise by the fusion of mononuclear precursors, and are "communal" cells of a sort, an osteoclast is technically referred to as a syncytium. (From Greek: "syn-" = same, and "kytos" = cell). Syncytial cells are found in other locations, and in general, syncytia have properties of invasiveness and capabilities for tissue destruction.
Osteoclasts specifically have the capacity to secrete hydrogen ions, pumping them out of the cytoplasm into the space between the osteoclast and the face of the bone. This lowers the local pH, and dissolves the mineral matrix. The osteoclast's other enzymes take care of the organic material. The word "osteoclast" is reflective of this cell's function: it comes from the Greek root klastos, meaning "to break." An "iconoclast" is (literally) one who "breaks" icons (and hence in modern English the word means anyone who challenges conventional ideas or institutions) and thus an "osteoclast" is "one who breaks bones." Very appropriate!
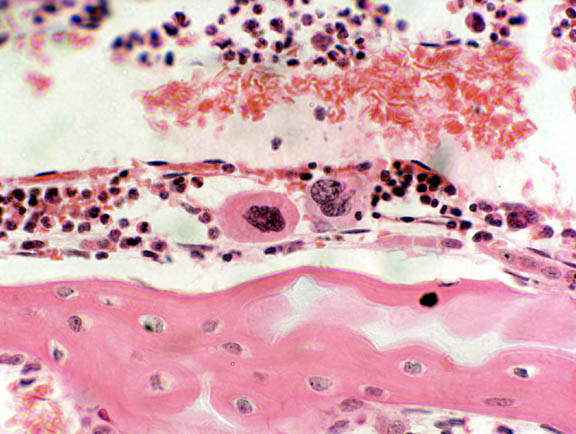
A cell often mistaken for the osteoclast is the megakaryocyte. This is actually a resident cell of the bone marrow proper, and it's the origin of the platelets of blood (see Exercise 6). A megakaryoctye is about the same size as the osteoclast, but if you came the one shown here to the osteoclasts above, you can easily see the differences. Megakaryocytes have large nuclei (hence the name) but they're aren't multinucleated. Two are shown here: the one on the right has a lobulated single nucleus, centrally located. Notice the position of the osteoclast's nuclei: off to one side of the cell, away from the "working" surface. The osteoclast is polarized, but the megakaryocyte isn't; neither is it syncytial or destructive.
The process by which bone is formed is terrifically complex in detail, and can't be with extensively here; but it's important to realize that bone always arises by the replacement of some pre-existing tissue. In some cases (i.e., the long bones) that pre-existing tissue is hyaline cartilage. The process is then called endochondral ossification ("endo"=within, and "chondro"=cartilage).
If instead of hyaline cartilage the pre-existing material is embryonic mesenchyme (primitive CT), as in the flat bones of the skull and a few other places, the process is called intramembranous ossification. These two modes are seen on slides 16 and 17 respectively.
The actual process of bone formation is the same in both cases. They differ in the material that's replaced, but the same cells and the same chemical reactions are involved. They aren't mutually exclusive, either: both can occur in close proximity.
The sequence of events by which a cartilage model is replaced by bone begins in centers of ossification set up in embryonic skeletons. Blood vessels invade the cartilaginous model, and carry into it the progenitors of bone-forming cells. Eventually three such centers are established: one at each end, and one in the center, of a long bone such as the femur or humerus. Throughout embryonic life the process of replacing the cartilage model with bone takes place; and at the time of birth most of the original model has been removed and replaced by bone. The principal function of hyaline cartilage, in fact, is to provide a "model skeleton" which will eventually be replaced by a new one made of bone. Note carefully that cartilage isn't "turned into bone," it's replaced. The old cartilaginous model is actually taken apart piece by piece while a new one is made in its stead.
This remarkable image shows the forming skeleton of an embryonic chicken, stained with Alizarin Red and Alcian Blue to differentiate between hardened bone (in red) and the remaining cartilage model (in blue). It is a beautiful example of how the process of endochondral ossification proceeds from the center of a long bone towards its ends. The proximal and distal ends of the femurs,the humeri, and the radii, for example, have not yet established their local secondary centers of ossification, but the shaft of all these bones is well along in the process. The beginnings of ossification can be seen in the pelvic girdle as stippled red areas. The mandible is pretty well complete, but the vertebrae are entirely collagenous.
It should be obvious that none of these bones are in their final form; as the embryo ages to become a hatchling and as the chick grows to adult size, all of the bones have to modify their length, shapes, and proportions. Bone development is a dynamic and on-going process. Even when the skeleton is fully matured and there is no more shaping and sculpting to be done, the removal and renewal of bone goes on. The necessity to maintain structural strength and physiological stability keeps bone an active, changing, growing tissue until the day the organism dies.
Now let's look at slide 16, demonstrating the process of endochondral ossification. Endochondral ossification doesn't end at birth; it can't, because it's the means by which bones grow in length as the animal ages. Lengthening of a bone occurs at the epiphyseal growth plates, which is the remnant of the cartilage model after nearly all the rest of the cartilage has been replaced. The cartilage in the growth plate is capable of proliferating in response to hormonal signals. Since the only location where this happens is at the ends of the bones, the bone can be extended in length for some considerable period of time after birth. In humans, the process of bone elongation doesn't end until the mid to late teen years. In domestic animals how long the process continues after birth varies: it takes about 18 months in dogs, longer in larger animals. What happens is that the growth plates are obliterated and disappear, after which no more elongation can take place.
Before discussing the process in some detail, one thing that should be noted is that a growth plate is always located between two centers of ossification. In other words, the wave of ossification is proceeding towards it from the center of the long bone and from the secondary center at the end. Activity in the secondary centers ends fairly soon, but the growth plate on the other side (next to the primary center) is proliferating like mad, keeping up with the simultaneous waves of bone formation. It's the balance between its ability to keep ahead of the ossification that's responsible for elongation.
By making cartilage faster on one side than the other, the growth plate "moves" towards the end of the bone and the bone elongates. Growth hormone (somatotropin) is a strong stimulant to cartilage growth, and as the animal grows, the secretion of GH keeps the growth plate active. But as the animal reaches puberty and GH production declines, the growth plate is unable to keep pace with the ossification process, and sooner or later it's obliterated. At that point elongation stops.
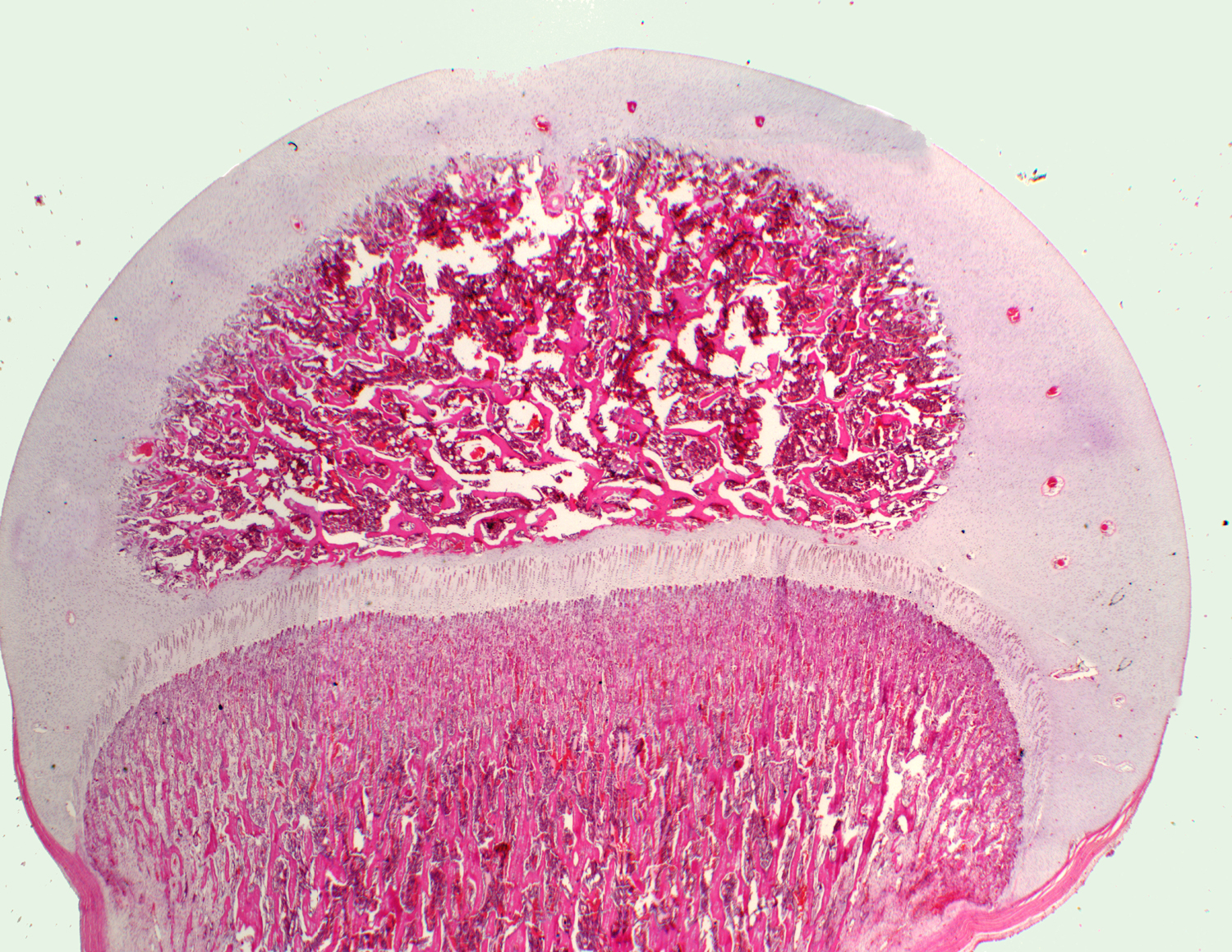
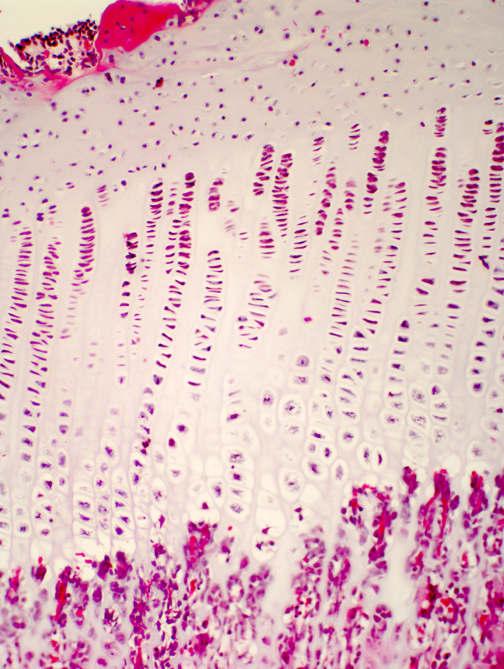 At right is the epiphysial region: scrolling the cursor over it will delineate the growth plate specifically. At left, some of the zones of the growth plate are shown in higher magnification: Scroll the cursor over the image at left, proceeding from the top, and you'll see some of these marked out; some more detailed images of the "leading edge" of the ossification follow below.
At right is the epiphysial region: scrolling the cursor over it will delineate the growth plate specifically. At left, some of the zones of the growth plate are shown in higher magnification: Scroll the cursor over the image at left, proceeding from the top, and you'll see some of these marked out; some more detailed images of the "leading edge" of the ossification follow below.
Ossification proceeds in an orderly manner. At one end of the cartilage is doing very little; this is the zone of resting cartilage. As you move towards the center of ossification, you will see an area in which the chondrocytes are proliferating: the spacing between isogenous groups is reduced, there are more cells per group, and they line themselves up into neat rows parallel to the long axis of the bone in this zone of proliferation.
In the next zone, the zone of hypertrophy and cell death, the chondrocytes undergo swelling and die: they've reached the end of the line and their fate is to provide a scaffold for the osteoblasts to work on to form the first generation of new ("woven," or "primary") bone.
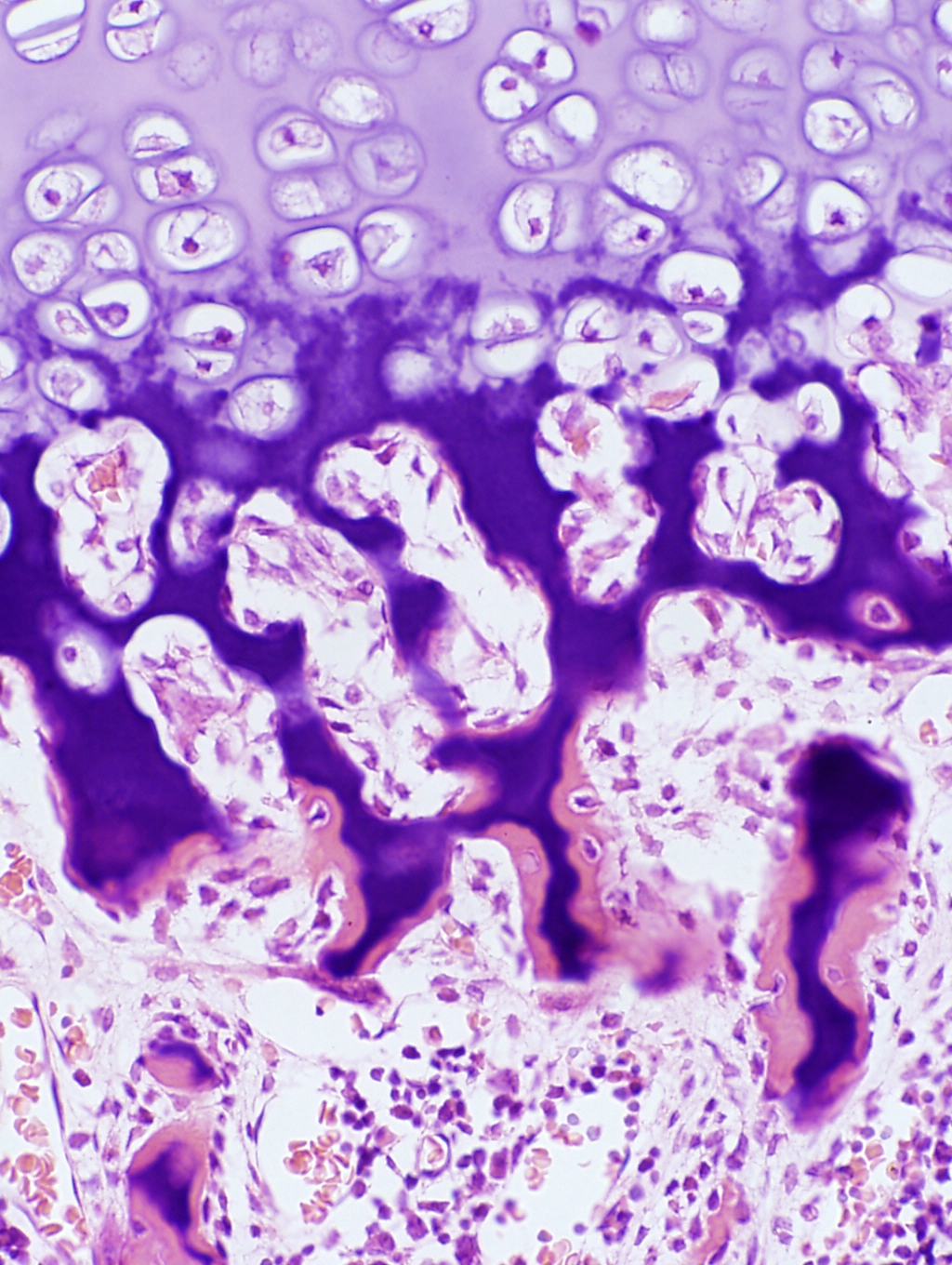 We haven't yet seen all the zones. Following the death of the chondrocytes, local changes in ionic state and pH trigger the deposition of calcium salts in the remnants of the cartilage matrix. This "leading edge" really can be broken into two zones, shown at left: a zone of provisional calcification It's called "provisional" calcification because it's really only a temporary state: the hardened matrix gives the initial groups of osteoblasts a place to work. Just as construction of a building requires a scaffold on which the builders can stand, so too does osteogenesis. At the edge of the hypertrophied area the lacunae burst open and become invaded by capillary loops and osteogenic cells from the marrow
cavity. The process of laying down bone on the calcified spicules of cartilage
begins.
We haven't yet seen all the zones. Following the death of the chondrocytes, local changes in ionic state and pH trigger the deposition of calcium salts in the remnants of the cartilage matrix. This "leading edge" really can be broken into two zones, shown at left: a zone of provisional calcification It's called "provisional" calcification because it's really only a temporary state: the hardened matrix gives the initial groups of osteoblasts a place to work. Just as construction of a building requires a scaffold on which the builders can stand, so too does osteogenesis. At the edge of the hypertrophied area the lacunae burst open and become invaded by capillary loops and osteogenic cells from the marrow
cavity. The process of laying down bone on the calcified spicules of cartilage
begins.
In the image at left you'll see that the hypertrophied cartilage is pale but the provisionally calcified cartilage has taken on a deep stain (the calcium salts bind the hematoxylin dye strongly). At the very edge, however, the pink color indicates that bone formation has begun.
Osteoblasts have come in and settled on the calcified matrix, and laid down osteoid the glycoprotein matrix material characteristic of bone. They then have begun to calcify that: you can see some of them are already building lacunae around themselves. This is primary or woven bone. It lacks the structural integrity and strength of lamellar bone, and it too is destined to be removed. Successive waves of osteoblasts and osteoclasts will remove the calcified matrix and the primary bone (whose fibers are randomly oriented) and lay down new, regularly oriented fibers, more osteoid, and then calcify that. The first generation of osteons will be constructed, by which time the process of initial osteogenesis as shown here will have moved on to a new location.
Slide 17 demonstrates intramembranous ossification, in which embryonic mesenchyme (rather than embryonic cartilage) is replaced by bone. This mode of ossification is typical of flat bones, such as some of those in the skull (e.g., the frontal, parietal, occipital, and temporal) and a few others. The initial stage begins when embryonic mesenchyme condenses into a very vascular CT, and mesenchymal cells produce a thin matrix material among the existing collagen fibers. Cells of the nearby CT differentiate into osteoblasts and become active in the secretion of additional bone matrix.

In intramembranous ossification, bone formation takes place at numerous sites simultaneously, one characteristic that distinguishes it from endochondral ossification. Eventually the separated areas will "grow together" as they expand: more collagen fibers are made, the production of matrix continues, and calcification of the matrix occurs, trapping the osteoblasts in their lacunae and causing their conversion to osteocytes.
In slide 17, which is a frontal bone, the ossified areas are stained rather darkly compared to the rest. In the example you see here, the irregular structures in the center of the image will form the spongy bone of the "sandwich" a typical flat bone displays in gross sections. The outer compact bone—the "tables" to use the gross anatomist's terminology—will be formed by ossification of the dense areas top and bottom in this field. This process hasn't yet begun in the specimen used for this example. Even though one region in this field will produce spongy bony and one compact bone, both arise via the intramembranous mode.
In intramembranous bone formation, the principal difference from endochondral ossification is what is being replaced. The tissue being replaced here is embryonic CT, not cartilage. Other than that, the events are the same, and the fully-differentiated cell types involved are the same. The two processes can occur side by side, and do, in the case of fracture repairs. Intramembranous ossification is also responsible for "shaping" the outer surfaces of many long bones. Intramembranous and endochondral ossification can, and do, occur simultaneously, so don't be confused by the presence of hyaline cartilage on slide 17.
Note also that while the growth of a long bone in length is done via the endochondral mode, intramembranous ossification also takes place on the surface as a way to maintain the proper shape and thickness of the anatomic bone. This can be seen on slide 16. The osteogenic potential of the precursor cells of the periosteum means that the bumps and humps on the surface (all the condyles, protuberances, origins and insertions you learned in Gross Anatomy, in other words) can be put in the right places and formed in the right shape. By controlling the rate of osteogenesis and the rate of osteolysis, the growing animal can reshape bones as needed by differential growth. Thus the final shape of the bone is determined by a combination of the two processes, exquisitely integrated and coordinated to produce exactly the right shapes.
One situation in which the process of ossification is galvanized into action in mature animals is in the repair of fractures. All of the cell types discussed above can be seen in fractures undergoing resolution. Both endochondral or membranous ossification are involved.