Objectives for This Exercise
Objectives for This Exercise
 Female Reproductive System
II: Avian
Female Reproductive System
II: Avian
PRE-RECORDED LECTURE FOR THIS EXERCISE
In this exercise we will consider the histology and the microscopic anatomy of the female reproductive tract of mammals. (The complementary organs in birds will be covered in a separate exercise.)
The female reproductive tract includes an egg-producing unit, the ovary, and a long tube through which the discharged ova must travel (and in which fertilization takes place), the uterine tube or oviduct. This latter organ can be subdivided into different regions, all of which are physically continuous. The uterine tube leads to the uterus, the organ peculiar to Eutherian (or "true", or "placental") mammals. It's in the uterus that gestation of the fetus occurs.
Following the normal period of development the term fetus is expelled through the cervix, which may be considered as a separate component of the system, and eventually through the vagina, the last component of the tract. We will consider each of these organs separately.

Begin with slide 154. This is an ovary, shown here in its entirety. You can easily make out a cortex and a medulla. The cortex is the outer portion, in which you'll find the developing follicles. The latter is the CT core, which carries blood vessels into the organ. Arteries and veins are seen in the medulla and the CT fold which supports it. The ovaries depend from suspensory ligaments whose CT serves as a route for blood vessels, lymphatics, and nerves to reach the parenchymal tissue. The medulla of the ovary is the continuation of the CT into the organ proper. The ovarian cortex includes those areas in which the oocytes are located in their follicles. The developing follicles are distributed throughout the cortex, and (in most species) release of the egg can occur at any point on the surface. At this magnification neither the tunica albuginea nor the overlying fold of peritoneum that covers the ovary can be made out.
Tunica Albuginea
The ovarian cortex is shot through with collagen fibers, continuous with those in the tunica albuginea. These fibers constitute the ovarian stroma, the framework which supports the ovarian parenchyma. The parenchyma is the "functional tissue," i.e., the follicles and the various interstitial cell types. (We will describe these shortly.) The stroma is essentially a ramification of the medulla, through which blood vessels enter and leave the cortex and surround the developing follicles.
 Follicles are surrounded and enmeshed in a webwork of collagen fibers, with fibroblasts and other cells in among them too.This image also shows quite clearly the extensive irregular CT that "fills the spaces" between the follicles. This is the ovarian stroma, and it's continuous with the CT of the medulla and the tunica albuginea. Some of the cells of the stroma are merely "maintenance" types for the fibers (i.e., they're basically fibroblasts). At left you see the cortical region of an ovary stained with the Masson's stain: the extent of the CT in the stroma is obvious. Some of the stromal elements, however, do have a role to play in the formation of late follicle stages. This will be discussed below. The stroma also contains innumerable small blood vessels. The scanning EM image above right is a corrosion cast to show the developing microvasculature around one follicle, most likely not beyond the secondary stage in this case (see below). In this specimen the extensive capillary network surrounding each developing follicle has been filled with plastic, and the tissue removed. As the follicle increases in size and its metabolic demands increase, the vascularization has to keep pace with it; if it doesn't the follicle will undergo regression (see below).
Follicles are surrounded and enmeshed in a webwork of collagen fibers, with fibroblasts and other cells in among them too.This image also shows quite clearly the extensive irregular CT that "fills the spaces" between the follicles. This is the ovarian stroma, and it's continuous with the CT of the medulla and the tunica albuginea. Some of the cells of the stroma are merely "maintenance" types for the fibers (i.e., they're basically fibroblasts). At left you see the cortical region of an ovary stained with the Masson's stain: the extent of the CT in the stroma is obvious. Some of the stromal elements, however, do have a role to play in the formation of late follicle stages. This will be discussed below. The stroma also contains innumerable small blood vessels. The scanning EM image above right is a corrosion cast to show the developing microvasculature around one follicle, most likely not beyond the secondary stage in this case (see below). In this specimen the extensive capillary network surrounding each developing follicle has been filled with plastic, and the tissue removed. As the follicle increases in size and its metabolic demands increase, the vascularization has to keep pace with it; if it doesn't the follicle will undergo regression (see below).
Ovarian Follicles & Their Fate
The individual follicles, each of which contains an egg which can potentially be ovulated, may be seen scattered through the cortex in various stages. These stages follow a distinct progression from the very earliest stage to the final, ovulated one; although in fact most never do ovulate. While in testes true "stem cells" (i.e., the spermatogonia) remain present and actively divide to maintain the supply of spermatozoa indefinitely, the situation in the ovary is very different: the stem cells of eggs, the oogonia, are not present in mammals at the time of birth.
In the course of embryonic development, all of the oogonia that are ever going to develop from the germ cell line do so, and then, as the ovary forms, further differentiate into the next stage, oocytes. There are two stages of oocyte development, and in primordial follicles, you will find not oogonia, but primary oocytes. These are arrested in development at the stage of first meiotic prophase, during life in utero. By the time of birth they have all reached this state of suspended animation, which lasts for years (or even decades in long lived animals).
Female mammals are therefore born with all the follicles they're ever going to have, and each one contains a primary oocyte. There are substantial numbers of these. In humans the total number of primordial follicles present at birth can easily reach 500,000 or more, "asleep" until the onset of puberty. Hormonal changes that occur at that time will cause them to "waken" from their dormancy and begin to develop. Only a few out of those hundreds of thousands do reach the stage of ovulation, or anything close to it. Humans have a much longer reproductive life than most other mammals, but even so, if a woman ovulates once a month from the ages of 12 to 50, she can produce only about 400-500 matured gametes capable of being fertilized. That's at best only one-tenth of one percent of her total.
Among large domestic animals, whose reproductive lifetimes rarely exceed a decade, obviously the number of naturally occurring ovulations is correspondingly smaller, though there may be as many follicles available to start with. Even in species which routinely produce litters, the "wastage" of follicles is very high: ff a bitch has as many as ten litters of 10 pups each (a highly unusual number for most breeds) she's only going to use four-one thousandths of a percent of her follicles. Thus, in all species only a minuscule fraction of those follicles will ever get past the primordial stage.
The vast excess of potentially fertilizable eggs is Nature's way of overcoming the very high mortality of embryos in utero and postpartum. By ensuring that there are always more eggs to be fertilized, any that are lost due to spontaneous abortions and any offspring that die from exposure, disease, starvation, predation, etc., can be replaced.
In this day and age of modern medicine, it's also the foundation for the technique of superovulation. Hormonal manipulation will cause many more follicles to develop and release eggs than would otherwise be the case. These can then be collected, inseminated in vitro, and distributed into surrogate "mothers" for development, a widely-used practice in cattle (and in humans). A very valuable cow can be superovulated and her oocytes collected, fertilized in vitro (often with frozen sperm from an equally valuable bull); they are then transferred to a less valuable surrogate mother, who will bear prize calves to which she has no genetic relationship.
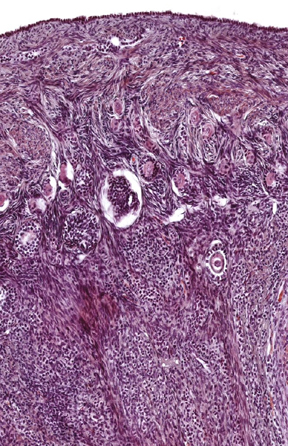
Primordial Follicles

In this image you see some primordial (P) and primary (1°) follicles, both early stages of development. The primordial follicle is the earliest stage, the one in which all follicles are at birth, and the one in which most of them die. The primordial follicle is characterized by a single layer of thin, squamous follicular epithelium around its periphery. The primary follicle, the next stage, also has a single layer of epithelium, but it's cuboidal in shape.
Primordial follicles will be located close up under the outer surface of the organ (i.e., right up near the tunica albuginea) usually occurring in clusters. They're grouped because when the oogonium divides and redivides, the daughter cells tend to remain in the same area. Within each primordial follicle is a primary oocyte (still diploid, because it's arrested in the course of its meiotic division, in first meiotic prophase) surrounded by a single layer of squamous follicular epithelial cells.
Primary Follicles
 Some primordial follicles do eventually progress to the next stage. Assuming that The Call comes, a primary follicle is formed. Several are shown at right. Notice how the follicular epithelium transitions from squamous to low cuboidal, though there remains only the one layer.
Some primordial follicles do eventually progress to the next stage. Assuming that The Call comes, a primary follicle is formed. Several are shown at right. Notice how the follicular epithelium transitions from squamous to low cuboidal, though there remains only the one layer.
In the image at right you see a couple of such, making the transition from primordial to primary. As development proceeds, the follicles in a given "nest" may develop more or less synchronously, as has happened here. The oocyte becomes metabolically active, and begins to synthesize new cytoplasm, causing it to enlarge compared to the oocyte in primordial follicles, but it is still a primary oocyte. There should be at least one such primary follicle in your slide, showing these features. Out in the ovarian stroma accommodation is being made to the changing conditions: cells that will become part of theca externa (see below) are becoming active and starting to proliferate.
Sooner or later, one follicle is going to outstrip the rest in the race to complete development. In this field there is one which has done so, and it's developed to the point where its follicular epithelium is getting stratified in places. Although this is not yet a definitive secondary follicle, it's advanced enough to be considered in the transition, so we'll designate it as "1o-2o" to indicate its status.
Always be aware that the progression of follicular development is a continuum; there is a smooth transition from one stage to the next, so you will expect to see follicles that are "intermediate" in appearance between one stage and its successor.
Secondary Follicles
 In the next stage, the follicular epithelium becomes distinctly stratified, and
the oocyte comes to develop a thick, translucent covering, the zona pellucida
(ZP). The ZP is an amorphous, non-cellular glycoprotein coat, which actually is a product
of the surrounding follicular epithelium. It serves as a sort of basal lamina
to the first layer of these cells, pressed up against the egg. Follicles demonstrating a stratified follicular epithelium, and enclosing
oocytes with a distinct ZP are secondary follicles. Even so, the oocyte
inside is still a primary oocyte.
In the next stage, the follicular epithelium becomes distinctly stratified, and
the oocyte comes to develop a thick, translucent covering, the zona pellucida
(ZP). The ZP is an amorphous, non-cellular glycoprotein coat, which actually is a product
of the surrounding follicular epithelium. It serves as a sort of basal lamina
to the first layer of these cells, pressed up against the egg. Follicles demonstrating a stratified follicular epithelium, and enclosing
oocytes with a distinct ZP are secondary follicles. Even so, the oocyte
inside is still a primary oocyte.
This is an early stage of the transition to a secondary follicle: the follicular epithelium has proliferated a bit, and layering is seen, more prominently on one side than the other.
Here's a much later stage, one that we can definitively refer to as a secondary follicle: the oocyte is greatly enlarged, the stratification of the follicular epithelium is obvious, and most importantly, the zona pellucida has been formed.
The ZP serves as a protective covering for the oocyte. It also participates in the formation of a barrier to polyspermy after ovulation. It's  produced by the follicular epithelial cells closest to the oocyte, as a sort of thickened basal lamina. Its presence here with the proliferated epithelium defines this as a secondary follicle.
produced by the follicular epithelial cells closest to the oocyte, as a sort of thickened basal lamina. Its presence here with the proliferated epithelium defines this as a secondary follicle.
Meanwhile, in the region outside of the developing follicle there are a few rows of "epithelioid" cells intermeshed with the stromal elements. These interstitial cells are forming the theca (from Greek, a "cup"). The thecal cells will eventually come to form a "wall" around the outside of this follicle.
I'm getting ahead of myself here, but need to mention that follicular epithelial cells are secretory: should this particular oocyte be The Chosen One that will ovulate, the follicular epithelial cells and the thecal cells derived from the stroma will participate in the formation of the corpus luteum, the temporary endocrine gland that will fill the space left when the follicle releases its oocyte.
 The follicular epithelium isn't static: it has an important role to play in the development of the follicle. As it proliferates and stratifies, it's also producing fluid and secreting it into the interstitial space. Eventually the turgidity of this fluid and the pressure it builds up get to the point where adjacent follicular cells are forced apart, causing fissures to form. These fissures will grow in size and coalesce as secretion continues. Here you see a secondary follicle undergoing this fissuring process, the initial step in the the transition of this follicle to the next stage of development. This is the early stage: the cavity is fairly small but will grow as more and more fluid is pumped into it. As the fluid forces the cells apart the antrum, the fluid-filled cavity that is the distinguishing
hallmark of the next stage is formed. This the tertiary or antral
follicle.
The follicular epithelium isn't static: it has an important role to play in the development of the follicle. As it proliferates and stratifies, it's also producing fluid and secreting it into the interstitial space. Eventually the turgidity of this fluid and the pressure it builds up get to the point where adjacent follicular cells are forced apart, causing fissures to form. These fissures will grow in size and coalesce as secretion continues. Here you see a secondary follicle undergoing this fissuring process, the initial step in the the transition of this follicle to the next stage of development. This is the early stage: the cavity is fairly small but will grow as more and more fluid is pumped into it. As the fluid forces the cells apart the antrum, the fluid-filled cavity that is the distinguishing
hallmark of the next stage is formed. This the tertiary or antral
follicle.  There should be several such follicles on this slide, of different sizes.
There should be several such follicles on this slide, of different sizes.
Note also the continued proliferation and development of the thecal cells around the outside of the follicle. By the time a follicle reaches this stage, the thecal cells are forming a distinct zone of their own. This antral follicle is more or less fully developed: from this point it may grow in size but its architecture won't change. You may correctly term any follicle enclosing a fluid filled antrum a tertiary follicle, whether you see an oocyte in it or not. The thecal cells should be easily discernible in the interstitial areas near late secondary follicles.
Cumulus Oophorus and Zona Granulosa
By the time the antral follicle is formed, the oocyte has become isolated onto a mound of (former) follicular cells. This mound is the cumulus oophorus (CO). A sub-population of cells in the CO is the single layer immediately adjacent to the oocyte and butting up against the ZP. This is rather elegantly termed the corona radiata (CR), a "glowing halo" of cells will go with the oocyte when it is ovulated. Thanks to the formation of the antrum, we can now also distinguish the wall of the follicle as a separate, thinned-out, stratified layer of follicular cells, quite distinct from the CO, though of course attached to it. Note that all of these subdivisions are derived from the former population of follicular epithelial cells. The "wall" of this follicle now consists of the membrana granulosa (G), around which theca is very well developed. If this follicle can continue to grow, it will eventually attain a size so large it causes a bulge on the surface of the ovary. When this happens the stage of a mature follicle has been reached and it's ready to release its oocyte.
Immunostaining of the wall of the follicle (right) for specific cell products shows that the theca (TC) and the granulosa cells (GC) are indeed two separate populations, though it's hard sometimes in routine preparations to make this out.
Mature Follicle
Maturation proceeds apace, and the follicle increases in size until it's a mature follicle (formerly called the "Graafian" follicle for Reijnier de Graaf, 1641-1673, a Dutch anatomist and physician). You probably won't see this stage on your slide, but a demonstration will be available. Once it reaches this stage, the follicle is ready to ovulate.
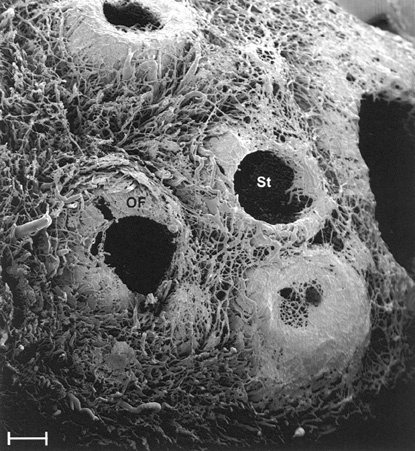 Post-Ovulation Events and Stages
Post-Ovulation Events and StagesThe mature follicle bulges out on the surface of the ovary: if ovulation is to occur, a necrotic plaque (the stigma) forms. At this location the surface cells lyse; the ripened oocyte with its accompanying corona radiata is cast loose from the rest of the cumulus oophorus, and it leaves the now ruptured follicle behind, as it begins The Immense Journey.
This striking scanning electron micrograph shows a corrosion cast specimen of several ovulated follicles, and the stigmata through which the oocyte emerged. The stigmata (St) are obvious: at least three of these four follicles ovulated. Note that in the one at lower right, however, some blood vessels remain in place: likely this one got all the way to the mature stage and then stopped developing (see below). This ovary came from a doe goat, a species which often has multiple ovulations and multiple births.
Corpus Hemorrhagicum
Turn now to slide 678. This is the first stage after ovulation. When ovulation occurs, the emptied follicle becomes filled with blood (there is a little hemorrhage involved in the process) and is termed a corpus hemorrhagicum, or "bloody body."
This section from slide 678 shows pretty dramatically why the term "hemorrhagicum" is applicable. In this field, about half the hemorrhage is still present as a distinct area. The location of the stigma would be in the thinned-out region on the right; and the left side is the forming corpus luteum.
The corpus hemorrhagicum is a transient structure with a
lifespan measured in hours rather than days. Once ovulation has taken place the cells of the theca interna, together with those of the zona
granulosa, collapse inward and proliferate to fill the empty space that was once a mature follicle's antrum. At high magnification you'll be able to see the "front" between the hemorrhage (being removed
by macrophages) and the proliferating cells behind it. The macrophages are filled with hemosiderin. The image at left is a high-power view of a CH stained with H&E (left) and the Prussian Blue reaction (right). The content of iron in these cells is very high as they absorb the clot and remove it so the CL can grow ever larger.
The cells of the corpus luteum—derived
partly from the theca and partly from the zona granulosa—are the source of the
steroid hormones that maintain the pregnancy. At right you can see a recently ovulated follicle: some remnants of the follicular fluid that filled the former antrum are present, the clot has been removed, and the wall of the old follicle is rapidly filling in the space that was once occupied by the follicle.
Corpus Luteum
The corpus luteum (CL) is a temporary endocrine organ. It produces progesterone. This process is about half completed on slide 678, but you will see a fully formed CL on a demonstration slide and in the image below. To form the CL, the proliferating endocrine tissue (the cells that used to be part of the cumulus oophorus, and the theca interna) is invaded by blood vessels from the medulla and stroma. In conjunction with vascularization, differentiation of the cells occurs and they become steroid producing units located next to blood vessels.
Eventually, even the most prominent CL faces a sad fate: t o become nothing more than a glassy remnant. After its useful life is finished, it eventually becomes a corpus albicans, or "white body."
Here's a few of the corpora albicantia (CA) or "white bodies." These are the glassy-looking scars that result from the degeneration of follicles that have ovulated. They result from the invasion of macrophages to clean up necrotic material and the proliferation of collagen fibers in the space. Some animals show these more prominently than others. Primates in particular have very obvious corpora albicantia, and so do sheep generally. You should be able to spot a good many of them on slide 154, which is from a monkey. In some species the scarring may be minimal and hard to see. In this field there's a large corpus albicans (CA) and several smaller ones, pretty typical for a primate ovary. The atretic follicle at lower right is in the process of degeneration and will produce a smaller and very similar scar when it's finished the process of degeneration.
What of those follicles which never develop, or whose development is arrested at
some point? The ripening of a follicle can be stopped at any point from
the primordial stage (which most follicles never leave) to the moment of
ovulation. Such follicles are termed atretic. If this does happen, the follicle regresses, the excess tissue is liquefied and
fluid absorbed, and CT invades the place where the follicle was, forming a
scar. This scar is a corpus atreticum. The distinction between a "corpus atreticum" and a "corpus albicans" is something of an academician's hair-splitting one: the corpus atreticum is a follicle that never ovulated, the corpus albicans has. In a good preparation, you can usually tell them apart by the presence of a small remnant of the zona pellucida in the corpus atreticum, as in this example. (Of course, if the follicle became atretic before the zona pellucida was formed, the remnant would not be present and no distinction could be made.) After enough time has passed it's hard—nearly impossible—to tell for sure what produced any given scar. So "corpus albicans" can really be applied to either situation, because after all, a scar is a scar.
The vast majority of follicles never make it beyond the primordial stage in the first place, and 99% never get past being a primary follicle. Atresia can occur at any phase of follicle development, up to and including the stage of maturity. It's perfectly possible for a follicle to reach the late antral stage and then suddenly cease its development and never ovulate at all: this is likely what happened to the one in the scanning EM image shown above.
The control of development of follicles and what signals one to complete development is very imperfectly understood. Obviously it involves hormonal signals, but there is also clearly some interplay among the follicles themselves. Dozens or even hundreds of follicles normally begin to ripen at the beginning of each cycle, but among all of those, only one (or at most a few) will complete the process. It's thought that signals produced by the follicle ripening fastest "turn off" or at least retard the development of others, perhaps using substances produced and diffused through the stroma by the thecal cells.
Well, at this point we've followed an oocyte to the moment it's been released, and it's on its way to meet its Destiny. Normally it's safely swept into the uterine tube by the ciliated epithelium of the infundibulum, the funnel shaped upper end. The infundibulum leads into the ampulla, the widest portion of the tube, seen on slide 684.
In the ampulla, the uterine tube is lined with simple ciliated cells, cuboidal to columnar in shape. In cross section, the mucosal lining is extremely convoluted, forming numerous folds and superfolds, a labyrinthine passageway through which the Intrepid Oocyte is propelled by the beating of the cilia.

This is the upper end of the tube, showing the finger-like fimbriae (left) that surround the infundibular opening. These projections are covered with ciliated epithelium over a core of CT. The cilia, by their beating, create a vortex that sweeps the newly-released oocyte into the upper end of the tube, and the cilia and the muscle in the tubule wall help propel it towards the uterus.
The ampulla, into which the infundibulum leads, is the longest and largest region of the uterine tube. Its mucosa is essentially the same as that of the infundibulum: a ciliated epithelium resting on a lamina propria of connective tissue. The muscular outer wall of the ampulla increases in robustness as you progress towards the uterus. It's made of smooth muscle, with strands running up into the mucosal folds. You can also see the CT investment of the tube, which is a fold of the peritoneum (the mesosalpinx, from the Latin for a trumpet).
 This is the ampulla region of the uterine tube, cut in cross section. As you can see, the lumen is filled with excursions of the mucosa, thrown up into extensive folds and subfolds that partially occlude the interior. They confront the egg with a tortuous and convoluted path to the lower part of the uterine tube and to the uterus proper. The image above and the one at right show the extensive branching and arborization of the mucosal folds, and the nature of the epithelium covering them. These are permanent structures.
This is the ampulla region of the uterine tube, cut in cross section. As you can see, the lumen is filled with excursions of the mucosa, thrown up into extensive folds and subfolds that partially occlude the interior. They confront the egg with a tortuous and convoluted path to the lower part of the uterine tube and to the uterus proper. The image above and the one at right show the extensive branching and arborization of the mucosal folds, and the nature of the epithelium covering them. These are permanent structures.
The mucosal epithelium in the ampulla (above, right) is a columnar ciliated sheet that may be pseudostratified in places. The oocyte (and, after fertilization, the zygote) is without means to move itself along, and so it's propelled by the beating of the cilia and the contractions of the muscular wall of the tube. Cilia were discovered in 1831 by the Swiss physiologist Gabriel G. Valentin (1810-1833) in the oviduct of the chicken.
Capillary beds in the lamina propria (seen in the image at right) provide for the cells' needs.
Why is the lumen of the ampulla so convoluted? The answer lies in the timing necessary between release of the oocyte and its readiness for implantation in the uterus. Fertilization in mammals actually takes place in the uterine tube, not in the uterus. By the time the zygote enters the uterus proper it has to be ready for implantation, so the initial stages of its development (cleavage) have to occur before it gets there. Hence the extraordinary development of the ampulla's mucosa: it's a means of slowing the oocyte's progress and precisely timing its arrival at the places where it's to be fertilized and its entry into the uterus.
Uterine Tube: Isthmus
The isthmus of the uterine tube is seen on slide 209, both in cross
section (left) and longitudinal section (below, right). The mucosal folds are much less complex than
in the ampulla, and the muscular wall is greatly thickened. The isthmus joins the ampulla and the uterus, and as the name implies, it's somewhat narrower in diameter than either.
The gross decrease in diameter is deceptive, however. As you will see here, the wall of the isthmus is considerably more muscular than that of the ampulla, and more importantly, its lumen is much more patent. The "star-shape" of the lumen in cross section is is due to longitudinal folds and an absence of obstructing convolutions.
The long straight passageway that the embryo has to traverse here imposes few if any obstacles, the extremely thick muscular wall provides plenty of propulsive force, and the folds guide the movement.
By the time the oocyte reaches the isthmus, it's not an oocyte anymore. It's been fertilized and is an early embryo, probably (in most species) having already undergone several cleavage stages. Since there's a limited time window for it to enter the uterus and implant before it dies, there is no longer any reason for delaying matters. Hence the large, open lumen and the muscular wall: both serve to propel that embryo into the uterus without any wasted time.
Slide 706 shows the final part of the isthmus, at the point where it enters the uterus; the isthmus is cut longitudinally, and appears to form a "tail" at one side of the section. The uterus has an even thicker wall than does the uterine tube, and the point of transition is marked by the presence of glands in the lamina propria of the uterine part of this specimen.
Uterus
Now examine slide 708. This is a section of uterus, and demonstrates most
of the features of the organ. The Greek for "uterus" is metra, so we speak of the endometrium to mean its lining of epithelium (and the associated connective tissue) and the myometrium to refer to its massive wall of smooth muscle. The myometrium is the largest single piece of smooth muscle in the body, and its size and extent can't really be appreciated in the microscope, certainly not in this field. The cranial end of the organ is also covered with
a layer of visceral peritoneum, the perimetrium, which has probably been
lost on your slide. The wall of the uterus—the myometrium—is arranged in several
layers (there are said to be up to seven, but I've never had much luck in
counting them).
The endometrium in the lumen of the uterus varies greatly in appearance depending on the stage of the estrus or menstrual cycle, but there are some consistent features. The lining epithelium in slide 708 is for the most part simple to pseudostratified columnar, and there are no ciliated cells. The lamina propria is very deep, and it's shot through with the profiles of the uterine glands, which produce material necessary for the support of the embryo and/or the capacitation of incoming sperm.
The histology of the uterus varies a great deal from one species to another, and most especially from one stage of pregnancy to the next; but some general statements can be made about it. The endometrium is a an epithelial covering over a rather extensive CT lamina propria. The epithelium is simple to stratified cuboidal, and the depth and extent of the lamina propria depend in large measure on the state of pregnancy. A consistent feature of the lamina propria is the presence of deep uterine glands. These glands provide nourishment for the early stages of embryonic growth, before the placenta is established. Structurally they're
simple tubular, though in late pregnancy they become very tortuous and coiled in shape. The degree of infolding and pocketing in the endometrium depends on the type of placenta that's formed, and how much interdigitation of the placenta with endometrium takes place.
The extensive uterine glands can be seen here. As you would also expect, there are many blood vessels in place among the fibers of the supporting lamina propria.
The height of the lamina propria, and the degree of coiling and proliferation of the glands is greatest when the uterus is being prepared for a Blessed Event. If the efforts of the reproductive apparatus come to naught, and no pregnancy results, the glands regress (though they are never lost entirely in most animals) and the height of the epithelium and lamina propria diminishes. On the next cycle, the organ, ever hopeful, does it all over again. In some primates, especially in humans, the covering is denuded right down to the myometrium, and sloughed in the menstrual flow.
Some appreciation for the stupendous complexity of the blood supply to the uterus can be gained from the striking image at left. This is a gross preparation of a "cleared" specimen of the uterus from a goat. In this technique the arteries are injected with plastic, and the tissue surrounding them rendered transparent by bathing the entire organ in oil of wintergreen for a few days. In this image the left and right uterine arteries (LUA and RUA) feed into ever more ramified branches that culminate in the capillary beds where the growing fetus will attach.
And of course...this is really only half the vasculature: the venous return isn't shown because it didn't get injected.
Cervix
The entrance to the uterine lumen is through the cervix, a cylindrical or
conical projection from the cranial end of the vaginal cavity. Seen grossly (left) the normal cervix has a small opening at the vaginal end, through which spermatozoa have to pass on their Quest. If you take a section through the cervix such that this cervical opening is centered, the histology is that of an extremely dense, heavily muscle-infiltrated wall with a great deal of CT in it. The wall consists of
little else but muscle and CT, making the cervix firm and rubbery on palpation. It's also capable of contraction
to close off its external orifice.
A longitudinal section through the cervical canal is shown at right. The channel is lined with epithelium, a simple cuboidal type, and the surface is folded to form cervical crypts.
The crypts have sub-crypts, whose lining epithelium is secretory.
When pregnancy occurs, it's important to seal the forming fetus up in a nice sterile chamber. The cervix is like a "stopper in the bottle" and except for the canal through its center, the uterine lumen is closed off from the outside world. The extensively reinforced fibromuscular wall contracts to close down the canal at the proper times (e.g. during pregnancy) to keep the fetus from popping out like a squeezed watermelon seed, and of course, it expands to permit passage of the fetus during birth. While contraction of the cervical wall shuts off most communication with the outside, microscopic openings would remain, if it weren't for the secretory cells lining the deep pockets and channels of the crypts. These cells produce a mucous material that hardens and seals off even the tiniest openings, prohibiting entry of bacteria and other deleterious materials into the uterine lumen. The formation of the cervical "plug" keeps the developing embryo in a nice sterile environment, maximizing its chances of survival.
Another function of the crypts is to present an "obstacle course" to the passage of sperm deposited in the vagina; the "weeding out" of the weak swimmers begins here. Only the strong survive.
Vagina
The cervix opens into the cranial end of the vagina. This is lined with stratified squamous epithelium and has glands embedded in its walls to provide lubrication during mating. A mucocutaneous junction is present where the lining meets the external integument of the body.
Picture Credits:
I am deeply indebted to two of my former students, some of whose work is included in this exercise. Dr Shireen Abdel-Gawad Hafez of the Department of Anatomy, Faculty of Veterinary Medicine, Alexandria University (Egypt) provided the scanning EM images and the stunning picture of the cleared uterine blood supply. Dr Amal Arafat Mouktahr of the Department of Histology, Faculty of Veterinary Medicine, Suez Canal University, Egypt, provided the immunolabeled images of the follicular wall and the Prussian Blue stained specimen of the macrophages of the forming CL.